Abstract
The first large randomized phase III trial in gene therapy demonstrated no improvement in the survival of patients injected with packaging cells that produced conventional replication-defective retroviral vectors carrying the herpes simplex virus thymidine kinase gene, a disappointing result that was attributed to extremely poor levels of transduction efficiency. To circumvent this problem, we have developed a modified replication-competent retrovirus (RCR) that is capable of transducing human glioma cell lines A-172, U-87, T-98G, U-373, and U-138 and rat glioma cell lines C6 and 9L, over multiple infection cycles in vitro, resulting in a tremendous enhancement in transduction efficiency over conventional replication-defective retroviral vectors at the same dose. Whereas the transduction efficiency of conventional retroviral vectors injected into pre-established subcutaneous U-87 tumors at a dose of 1.0 × 105 transducing units (TU) was only 0.2% at 6 weeks postinjection, the same dose of RCR vector resulted in up to 97.2% transduction. When RCR vectors at a dose of 1.0 × 104 TU were injected into pre-established intracranial U-87 tumors, transduction efficiency at 2 and 3 weeks was 74 and 98.1%, respectively. Notably, however, intracranial injection of RCR vectors did not result in detectable infection of normal brain cells. Furthermore, using a sensitive polymerase chain reaction assay, no detectable RCR signal could be observed in any extracerebral tissues, including lung, liver, kidney, upper gastrointestinal tract (esophagus and stomach), lower gastrointestinal tract (colon and small intestine), skin, spleen, and bone marrow. Treatment of U-87 intracranial gliomas with RCR vectors carrying the yeast cytosine deaminase suicide gene followed by 5-fluorocytosine prodrug administration resulted in 100% survival over a 60-day follow-up period, compared with 0% survival of control groups receiving vector alone or prodrug alone. Our results demonstrate that RCR vectors can achieve therapeutically significant levels of transduction in malignant human gliomas, and that RCR vector spread after intratumoral injection is restricted to the tumor itself.
OVERVIEW SUMMARY
Previous studies using conventional replication-defective murine leukemia virus (MuLV)-based vectors have reported extremely low levels of tumor transduction in vivo, frequently less than 0.1–1%. To circumvent this obstacle, we have developed replication-competent retrovirus (RCR) vectors that can efficiently transmit inserted transgenes not only in culture, but also throughout entire subcutaneous and intracranial gliomas in vivo. As MuLV is unable to infect quiescent normal cells, gene transfer appears to be highly restricted to the tumor itself without any detectable spread to normal tissues. Significantly improved survival was observed after RCR vector-mediated suicide gene therapy in intracranial glioma models.
INTRODUCTION
Glioblastoma multiforme (GBM), also known as a grade IV malignant glioma, is the most common and malignant form of primary brain tumor in adults (Chen et al., 1995). GBM tends to extensively infiltrate surrounding normal brain tissue, making complete removal by surgical means virtually impossible, necessitating other forms of therapy. However, despite major improvements in neuroimaging, chemotherapy, radiation treatment, and supportive care, these tumors have been found to be highly resistant to conventional treatment (Smiley et al., 1997; Leweke et al., 1998; Shu et al., 1998; Osmak et al., 1999). The overall prognosis for GBMs has changed little in the past two decades, and the average survival time of a newly diagnosed patient remains on the order of 12–15 months (Ma et al., 2002). The lack of effective treatment options and the extremely poor prognosis of this disease therefore necessitate the development of new therapeutic approaches such as gene therapy.
The first randomized major phase III clinical trial in gene therapy involved treatment of GBM patients by surgical resection followed by injection of irradiated PA317 packaging cells producing a replication-defective retrovirus vector containing the herpes simplex virus thymidine kinase gene (HSV-TK), and subsequent administration of the prodrug ganciclovir (GCV). Unfortunately, no significant difference in survival was found between patients treated with gene therapy versus control patients (Rainov, 2000), a result attributable to the extremely low efficiency of gene transfer reported in this study (Stuhlmann et al., 1989). More efficient transduction may be achieved if replication-competent viruses are used, as the virus would replicate and multiply after the initial infection event and each infected tumor cell would in effect become a virus producer cell.
A number of replicating oncolytic viruses are already being applied to the treatment of malignant gliomas, including adenovirus, herpesvirus, and poliovirus (Mandl et al., 2001; Nanda et al., 2001; Varghese et al., 2001). We have recently developed murine leukemia virus (MuLV)-based replication-competent retrovirus (RCR) vectors for cancer gene therapy, and we propose that these have significant advantages over other viruses as tumor-selective agents (Logg et al., 2001b). As MuLV contains no nuclear localization signals in its capsid and can infect only cells that are actively dividing, MuLV would be particularly advantageous in the context of gliomas, because adjacent normal neuronal cells are postmitotic. Furthermore, MuLV may not elicit chronic persistent inflammatory responses or encephalopathies, as might occur in the case of adenovirus and herpesvirus.
Here, we investigated the utility of MuLV-based RCR vectors in achieving highly efficient and tumor-selective gene transfer to glioma cells in culture and in vivo, and in achieving enhanced survival in application to suicide gene therapy in an intracranial glioma model.
MATERIALS AND METHODS
Construction of RCR vectors carrying green fluorescent protein and yeast cytosine deaminase transgenes
The replication-competent amphotropic MuLV vector construct, pAZE-GFP, containing an encephalomyocarditis internal ribosome entry site (IRES)-green fluorescent protein (GFP) cassette positioned between the env gene and 39 long terminal repeat (LTR), has been previously described (Logg et al., 2001a). The 59 LTR U3 region of pAZE-GFP was replaced with the cytomegalovirus promoter, generating pACE-GFP. Figure 1A shows a schematic representation of ACE-GFP, the replication-competent provirus portion of the pACE-GFP plasmid.
FIG. 1.
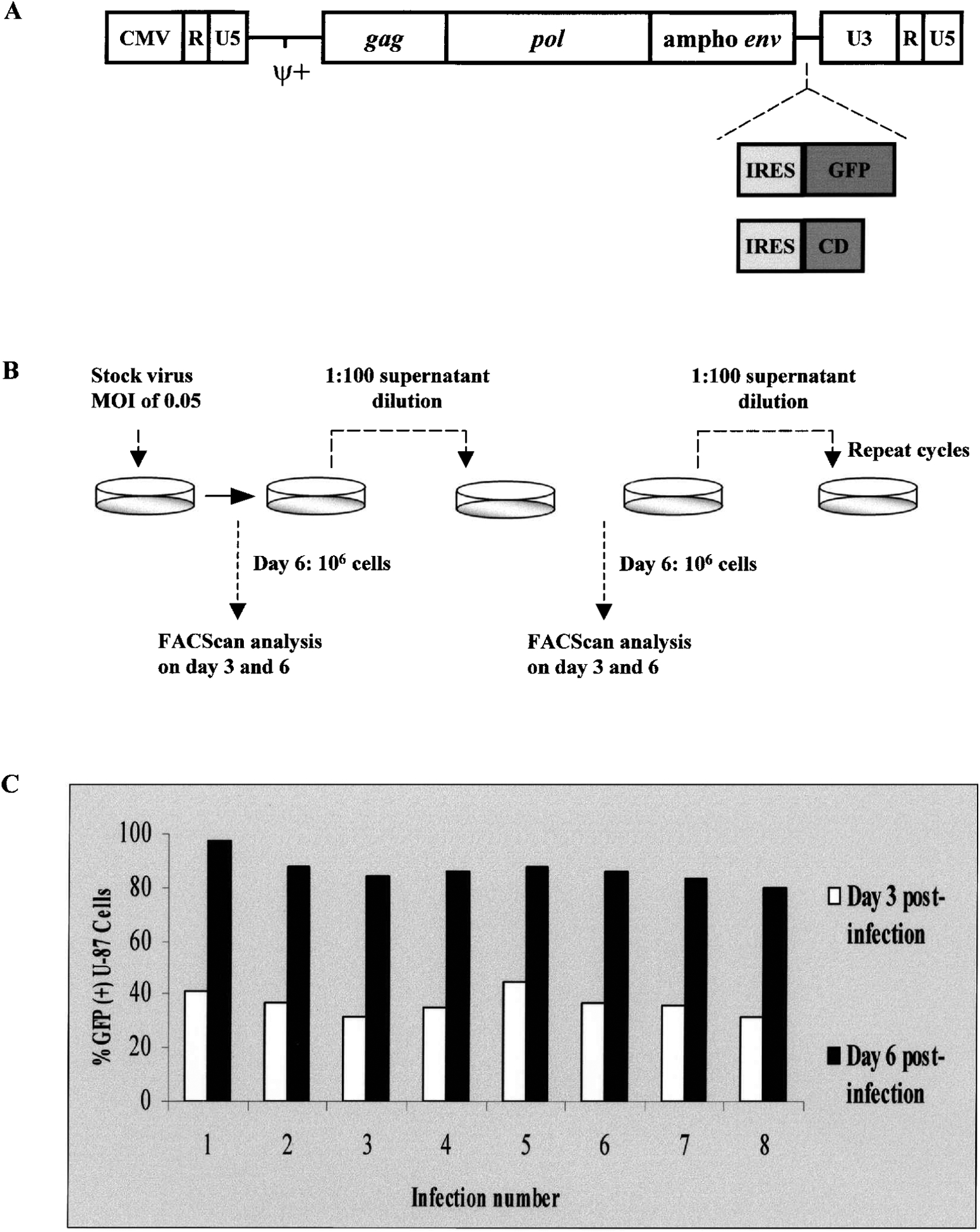
(A) Structure of replication-competent retrovirus vector proviral constructs carrying green fluorescent protein (GFP) and yeast cytosine deaminase (CD) transgenes. The Moloney murine leukemia virus (MuLV) proviral genomic sequence was modified by replacement of the U3 region in the 5’ long terminal repeat (LTR) with the cytomegalovirus (CMV) promoter, and replacement of the ecotropic envelope gene with the amphotropic envelope (env) from 4070A. Expression cassettes consisting of the GFP and CD transgenes preceded by an internal ribosome entry site (IRES) were inserted precisely at the boundary between the env and 3’ untranslated region (UTR) sequences of the modified MuLV genome. (B) Strategy for testing genomic stability of RCR vector in glioma cells. After production of the RCR vector ACE-GFP, containing the IRES-GFP cassette, by transient transfection of 293T cells, the supernatant medium was filtered, diluted 10-fold, and used for inoculation of a fresh plate of U-87 human glioma cells. On day 3 and day 6 postinoculation, as the cultures approached confluency, the cells were trypsinized and replated at lower density, and an aliquot was analyzed for GFP fluorescence by FACS. Supernatant medium from day 6 cultures was again harvested, filtered, diluted 100-fold, and used to inoculate another fresh plate of U-87 cells. These infection cycles were repeated multiple times. (C) Consistency of transgene transmission through multiple cycle infections with replication-competent vector ACE-GFP. The graph indicates the percentage of GFP-positive cells as determined by FACS analysis on day 3 and day 6 of each infection cycle, as described above. By day 6 of each infection cycle, the percentage of GFP-positive cells was consistently found to be greater than 80%.
Plasmid pCR-Blunt-CD, which contains the IRES-CD cassette, was kindly provided by P. Roy-Burman (Department of Pathology, University of Southern California, Los Angeles, CA). Using this plasmid as a template, the IRES-CD cassette was amplified by polymerase chain reaction (PCR), using the following primers:
5 ‘-TAACGTACGTTACTGGCCGAAGC-3 ’
5 ‘-AAGCGGCCGCCTACTCACCAATATCTTCA-3 ’
The PCR product was digested with the appropriate restriction enzymes and ligated into the RCR vector plasmid, replacing the IRES-GFP cassette. The proviral portion of the resultant vector, designated ACE-CD, is also shown in Fig. 1A.
Cell culture and viral vector production
Human glioma cell lines U-87, A-172, T-98G, and U-138, rat glioma cell line 9L, and human 293T cells (DuBridge et al., 1987) were grown in Dulbecco’s modified Eagle’s medium (DMEM) and supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified atmosphere of 5% CO2. Rat glioma cell line C6 was grown in RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified atmosphere of 5% CO2. For production of RCR vector stocks by transient transfection, LipofectAMINE PLUS reagent (Invitrogen Life Technologies, Carlsbad, CA) was used to transfect 1 3 105 293T cells with 5 mg of pACE-GFP or pACE-CD, producing replication-competent retrovirus vectors ACE-GFP and ACE-CD, respectively. For production of conventional replication-defective retrovirus vectors expressing GFP (RDR-GFP), 5 mg each of plasmid pCGP (expressing MuLV gag-pol), pCAE (encoding amphotropic 4070A env), and transfer vector pMLV-GFP were cotransfected as previously described by Cannon et al. (1996). The conditioned medium was harvested 48 hr posttransfection, passed through a 0.45-mm pore size syringe filter, frozen at 280°C, and titered before use. For transduction of glioma cells in culture, Poly-brene (4 mg/ml; Sigma, St. Louis, MO) was added to the supernatant medium at the time of infection.
Multiple cycle infections with replication-competent vector
Virus stock at a multiplicity of infection (MOI) of 0.05 was used to infect U-87 cells at 20% confluency. Three days postinfection, the cells were split 1:5 and an aliquot was analyzed for GFP expression by fluorescence-activated cell sorting (FACS) analysis. Six days postinfection, the cells were again analyzed for GFP expression, and the culture supernatant was diluted 100-fold and used to infect a fresh population of U-87 cells at 20% confluency. This cycle was repeated several additional times (Fig. 1B).
Retroviral vector titer determination
Serial dilutions of viral supernatant in a total volume of 1 ml culture medium were added to U-87 cells at 20% confluency in six-well plates. Forty-eight hours postinfection, the cells were incubated in medium with 50 μM 3’-azido-39-deoxythymidine (AZT) for 24 hr and subjected to FACS analysis to quantitate GFP fluorescence. The viral titer was calculated according to the formula: transducing units (TU)/ml 5 (number of cells counted immediately before infection × percentage of transduced cells reported from FACS analysis)/dilution factor of viral supernatant.
Cytospin cell preparation
After aspirating the supernatant medium, cells were washed twice with phosphate-buffered saline (PBS), trypsinized, and centrifuged at 1000 rpm for 5 min. Cells were resuspended at a density of 2 × 105/ml, and 200 ml of the cell suspension was added to the sample chamber of a Cytospin apparatus (Shandon, Pittsburgh, PA) and then spun at 500 rpm for 5 min with filtration. Cell preparations were either frozen at 220°C for future use or dried overnight for immunohistochemical staining.
In vivo studies
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern California. All mice were maintained in a pathogen-free environment throughout the experiment.
For normal brain studies, either 10 ml of PBS vehicle or ACE-GFP vector (1.2 × 104 TU/10 ml) was injected into the frontal lobe of normal Sprague-Dawley rats, using the following coordinates: 1 mm anterior and 3 mm lateral relative to the bregma, and 5 mm deep into the brain.
In all marker gene studies in tumor models, three animal groups (PBS control, RDR-GFP, and ACE-GFP) were employed. For subcutaneous and intracerebral tumor studies, 4- to 6-week-old male athymic nu/nu mice (weight, 20–30 g; Harlan Sprague Dawley, Indianapolis, IN) were inoculated with the human glioma tumor cell line U-87. For subcutaneous tumor models, 5 × 105 U-87 cells were injected into the right dorsal flank. For intracranial tumor models, 2 × 105 U-87 cells were injected into the right frontal lobe, 1 mm anterior and 1.5 mm lateral relative to the bregma, and 2.8 mm deep into the brain.
For the subcutaneous tumor model, when the U-87 xenografts were about 4–5 mm in diameter, 100 ml of PBS vehicle control, replication-defective retroviral vector RDR-GFP (1.0 × 105 TU/100 ml), or replication-competent retrovirus vector ACE-GFP (1.2 × 105 TU/100 ml) was injected into the cen-ter of each tumor. For the intracranial tumor model, 1 week after U-87 tumor inoculation, 10 ml of PBS vehicle control, RDR-GFP (1.0 × 104 TU/10 ml), or ACE-GFP (1.2 × 104 TU/10 ml) was stereotactically injected into the brain tumor with a 26-gauge Hamilton syringe (Martuza et al., 1991). For survival experiments using the intracranial model, one group of animals was stereotactically injected with 10 ml of PBS vehicle control and two groups were injected with ACE-CD (approximately 1.0 × 104 TU/10 ml); 8 days later, the PBS group and one of the ACE-CD groups subsequently received daily intraperitoneal injections of 5-fluorocytosine (5-FC; 500 mg/kg per day) for 15 consecutive days.
Tissue harvest and FACS analysis
Subcutaneous tumor models.
Subsets of tumor-bearing mice were killed by pentobarbital overdose at different time points: 2, 4, and 6 weeks after vector infection. Tumors were dissected under sterile conditions and randomly divided into four to six pieces. Three or more tumor pieces were immediately frozen at −80°C, along with other tissues including samples from normal brain, lung, liver, kidney, upper gastrointestinal (GI) tract (esophagus and stomach) and lower GI tract (colon and small intestine), skin, spleen, and bone marrow for later sectioning and immunohistochemistry. Randomly selected tumor samples were placed in tissue culture dishes, extraneous tissue was removed with a sterile scalpel, and samples were washed once with cold Hanks’ balanced salt solution (HBSS), minced into small pieces of about 1 mm in size, and digested with 0.2% collagenase/dispase (Roche Diagnostics, Indianapolis, IN) in HBSS in a 37°C water bath for 2 hr. The cells were pipetted up and down every 30 min to promote dissociation. The dissociated cells were then spun down by low-speed centrifugation (1000 rpm for 3 min), resuspended in PBS, and passed through a 100-um pore size nylon cell strainer (BD Discovery Labware, Franklin Lakes, NJ). Half the cells were plated onto culture flasks for explant culture. The remaining cells were immediately taken to the University of Southern California Flow Cytometry Laboratory for GFP expression analysis, using a FACScan (BD Immunocytometry Systems, San Jose, CA), using both FL1 (green) and FL2 (red) channels to identify and quantitate true GFP-positive cells. The advantage of this two-dimensional gating method is that even a few GFP-positive cells can be visualized inside the FL1+FL2− gate.
Intracerebral tumor models.
Animals were killed 2 and 3 weeks after ACE-GFP vector inoculation. The same procedures were performed as described above for tissue harvest, sample preparation and storage, and FACS analysis, except that because of the high percentage of positive cells, only single-parameter analysis based on the FL1 channel was used.
Immunohistochemistry
Sodium pentobarbital (100 mg/kg) solution was injected intraperitoneally and the animals were killed. The brains were taken out, embedded in O.C.T. medium, and frozen at −80°C. Fresh frozen tumor samples were sectioned at a thickness of 5 mm with a cryostat. All tumors were sectioned through their largest diameter, and the representative sections were used for immunohistochemistry. Briefly, sections were fixed in acetone for 5 min, dried for 10 min, quenched in 0.3% H2O2 for 5 min, and washed three times in cold PBS. Nonspecific binding was preblocked for 30 min at room temperature. Sections were incubated overnight at 4°C with a 1:200 dilution of rabbit anti-GFP polyclonal antibody (BD Clontech, Palo Alto, CA), and then incubated with goat anti-rabbit secondary antibody at a 1:200 dilution at room temperature after three washes in PBS. Immunoreactivity was visualized with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA), using 3-amino-9-ethyl-carbazole as a chromogen, and counterstained with hema-toxylin.
Genomic DNA isolation from tumor and organs
Genomic DNA from all frozen tumor and organ samples, including normal brain, lung, liver, kidney, upper GI tract (esophagus and stomach) and lower GI tract (colon and small intestine), skin, spleen, and bone marrow, was saved at −80°C and was isolated with a GenomicPrep cell and tissue DNA isolation kit (Amersham Biosciences, Piscataway, NJ). Frozen tissues were thawed and minced with sterile disposable scalpels. The minced tissues were lysed and homogenized on ice in cell lysis solution, and then the lysate was incubated at 65°C for 1 hr. After RNase treatment, protein precipitation solution was added. Isopropanol was used to precipitate DNA, which was washed in 70% ethanol and air dried. The genomic DNA was eluted in DNA hydration solution, and stored at 2–8°C. The total yield of DNA from each sample was about 10 μg.
PCR assay of GFP expression in tumor and organs
A genomic PCR assay, which amplifies an approximately 700-bp fragment of the GFP transgene, was performed to examine transduction and vector spread in subcutaneous and intracerebral tumors as well as various extratumoral organs. Briefly, amplification was performed in a reaction volume of 25 ml under the following conditions: 0.5 μg sample DNA, 0.2 μM dNTPs, 2.0 mM MgCl2, 0.5 μM of each primer, 1x PCR buffer without Mg, and 5 units Taq polymerase (Invitrogen). Products were amplified by 40 cycles of successive incubation at 94°C for 1 min, 57°C for 45 sec, and 72°C for 1 min, respectively. Amplification cycles were then followed by 5-min extension at 72°C and hold at 4°C. The primers used to amplify the GFP transgene were as follows:
5’ primer: 5’-AAGGGCGAGGAGCTGTTC-3 ’
3’ primer: 5’-TACTTGTACAGCTCGTCCATGC-3 ’
The same procedures were applied to amplify a 525-bp fragment of mouse b-casein DNA as an internal control, using the following primers:
5’ primer: 59-GATGTGCTCCAGGCTAAAGTT-3 ’
3’ primer: 5’-AGAAACGGAATGTTGTGGAGT-3 ’
After PCR amplification, the reaction products were loaded on 1.1% agarose gels and run at 70 V for about 1 hr, and visualized by ethidium bromide staining.
Statistical analysis
Survival data were analyzed according to the method of Kaplan–Meier, using SAS software (SAS Institute, Cary, NC) to calculate significance values. p Values of <0.05 were considered significant.
RESULTS
RCR vector-mediated transmission of the GFP transgene is stable for multiple cycles in glioma cells in culture
Replication-competent MuLV-based vectors containing an internal ribosome entry site (IRES) and transgene cassette inserted just between the envelope gene and the 3’ untranslated region (UTR) (Fig. 1A) have been found to exhibit greatly improved functional and genomic stability (Logg et al., 2001a) compared with all other reported MuLV-based RCR vector designs, which used transgene insertion positions within the 39 LTR U3 region (Reik et al., 1985; Stuhlmann et al., 1989; Varela-Echavarria et al., 1993; Coulombe et al., 1996). The RCR vectors used in the present study, ACE-GFP and ACE-CD, also contain an IRES-transgene cassette at the env-3’ UTR boundary, with one additional modification: a CMV promoter replaces the U3 region of the 5’ LTR to increase the initial transcription level of the RCR vector genome (Fig. 1A), thus increasing titers obtained after production by transient transfection of the vector genome into 293T cells. After the first round of reverse transcription, however, the U3 sequence from the 3’ LTR is reduplicated at the 5’ LTR, which thus reverts to the original wild-type MuLV sequence in all subsequent cycles of virus infection.
We have previously monitored the genomic stability of RCR vectors containing IRES-GFP marker gene cassettes over multiple passages by Southern blot analysis as well as genomic PCR of proviral DNA to detect any recombination or deletion events, and have confirmed that stability of GFP expression by FACS is a reliable surrogate marker for the structural stability of the RCR vector genome (Logg et al., 2001a). We therefore confirmed the stability of this newly modified RCR vector design by monitoring GFP transgene expression over time as the ACE-GFP vector spread through cultures of U-87 glioma cells. As previously, virus stock was prepared by transient transfection of 293T cells, and the supernatant medium was harvested and filtered through a 0.45-μm pore size filter. The titer of virus stock was determined to be 1.2 × 106 TU/ml by FACS analysis in the presence of AZT to prevent further replication. The spread of the vector was monitored by serial passage in fresh cultures of 2 × 105 U-87 cells infected with virus stock (MOI of 0.05). On day 3 and day 6 postinoculation, the infected cells were trypsinized and washed, and an aliquot was resuspended in PBS for FACS analysis to quantitate the percentage of GFP-positive glioma cells (Fig. 1B).
With an initial MOI of 0.05, initial transduction levels are low; although the percentage of GFP-positive cells at 48 hr was generally about 5 to 7% (data not shown), expression in the culture rapidly increased over time, so that 40% of the cells were GFP positive by day 3, and by day 6 the percentage of the culture expressing GFP approached 100% (Fig. 1C). At each time point, the cells reached 100% confluency and further virus spread was not observed unless the cells were trypsinized and replated at lower density. Thus, the FACS results exhibit classic viral replication kinetics, with a lag phase over the first 2 days, followed by a logarithmic increase in GFP expression as vector replicates through the culture over days 3 to 6, reaching the plateau phase as the entire cell population is transduced.
In subsequent infection cycles, 100-fold dilutions of conditioned medium from cells infected in the previous passage were employed, and we monitored the stability of GFP marker gene transmission over more than 8 serial infection cycles of the virus by FACS. In each cycle, transduction efficiencies consistently exceeded 80% by day 6; thus, the transgene is efficiently delivered throughout each fresh culture over multiple serial passages (Fig. 1C). Similar results were obtained for A-172, T-98G, U-373, and U-138 human glioma cells, as well as for C6 and 9L rat glioma cells (data not shown). Because each infection cycle was initiated with an aliquot of culture supernatant at 1:100 dilution, and the replicating vectors ultimately spread to approximately 106 U-87 glioma cells by day 6 in each cycle, for up to at least 7 cycles, and because each of the 99 other aliquots of diluted supernatant from each of the 7 cycles can also be used in this manner, this represents the equivalent of infection and gene delivery to 1007 × 106 = 1020 glioma cells without significant loss of vector stability.
ACE-GFP vector directly injected into normal rat brain does not transduce quiescent normal cells
As MuLV cannot infect quiescent nondividing cells, the RCR vector should similarly be largely incapable of infecting normal adult brain. Thus, to determine whether any transduction of normal brain tissue occurs with the RCR vector, 1.2 × 104 TU of ACE-GFP in a volume of 10 μl was injected into the right frontal lobe of normal Sprague-Dawley rats, and GFP expression was assessed by immunohistochemistry. No GFP signal was detected in the brains of rats injected with saline alone or with ACE-GFP (data not shown), confirming that ACE-GFP does not transduce normal brain cells.
ACE-GFP shows highly efficient and progressive transduction of U-87 subcutaneous gliomas
To determine the ability of ACE-GFP to achieve efficient transgene delivery in gliomas in vivo, U-87 human glioma cells (5 × 105 cells) were first implanted subcutaneously into athymic nu/nu mice. Tumors were allowed to grow up to 4–5 mm in diameter and then PBS vehicle control, ACE-GFP (1.2 × 105 TU/100 μl), or RDR-GFP (1.0 × 105 TU/100 μl) was injected into the tumor. After sacrifice at serial time intervals of 2, 4, and 6 weeks after vector inoculation, quantitation of GFP expression in the ACE-GFP- and RDR-GFP-transduced tumors was performed by FACS analysis immediately after fresh tumor dissection and digestion.
As expected, the percentage of GFP-positive cells in the freshly dissected tumors that had been infected with RDR-GFP was low (1.2% at 2 weeks and 0.2% at 6 weeks) after vector injection (Fig. 2A–C). In contrast, the percentage of GFP-positive cells in ACE-GFP-infected tumors was 70.6, 90.2, and 97.2% at 2, 4, and 6 weeks postinjection, respectively (Fig. 2D–F), and these FACS results were visually confirmed by fluorescence microscopy of the freshly dissected tumor cells (Fig. 2G and H). The results demonstrate that, in contrast to conventional RDR-GFP, the replication-competent ACE-GFP vector was capable of almost complete transduction of the entire U-87 tumor mass within 6 weeks.
FIG. 2.
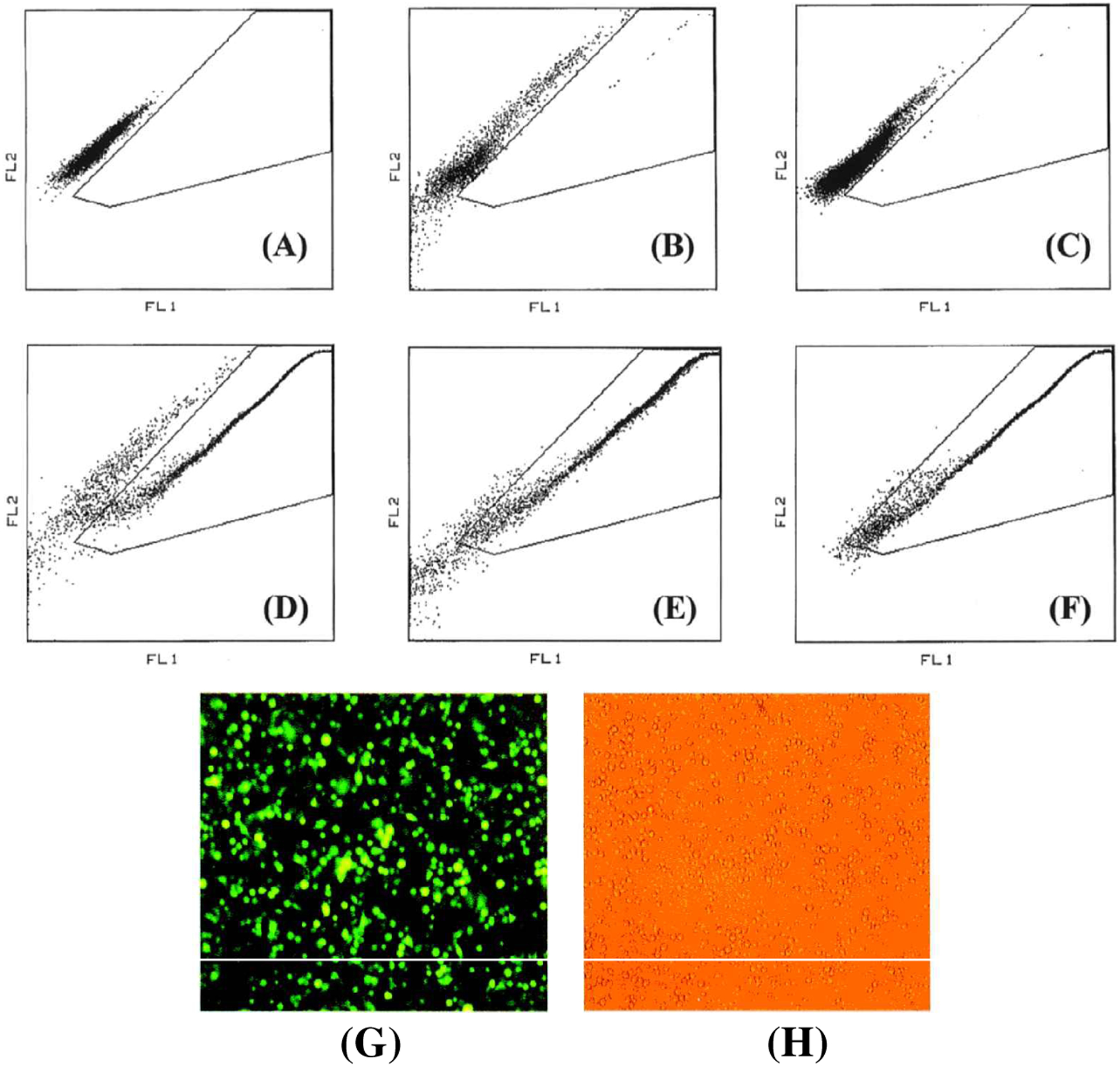
FACS analysis of ACE-GFP-transduced versus RDR-GFP-transduced subcutaneous gliomas. The indicated gate is calibrated for GFP-positive events, which deviate more toward the green fluorescence (FL1) channel (x axis) and away from the red (FL2) channel (y axis). (A) Untransduced control U-87 cells; (B) U-87 tumor, 2 weeks postinfection with RDR-GFP, shows 1.2% GFP+ cells; (C) U-87 tumor, 6 weeks postinfection with RDR-GFP, shows 0.2% GFP+ cells; (D) U-87 tumor, 2 weeks postinfection with ACE-GFP, shows 70.6% GFP+ cells; (E) U-87 tumor, 4 weeks postinfection with ACE-GFP, shows 90.2%GFP+ cells; (F) U-87 tumor, 6 weeks postinfection with ACE-GFP, shows 97.2% GFP+ cells. (G and H) Fluorescence (G) and visible light (H) micrographs of U-87 subcutaneous glioma cells immediately after tumor dissection and cell disaggregation, 4 weeks posttransduction, showing almost 100% transduction with GFP.
ACE-GFP also efficiently transduces intracerebral U-87 gliomas
For the intracranial tumor model, 2 × 105 U-87 cells were stereotactically implanted in the right frontal lobe as described above and, 7 days later, 10 μl of PBS vehicle or ACE-GFP (approximately 1.2 × 104 TU [total dose]/10 μl) was injected into the tumor, using the same coordinates. Immunohistochemical staining with GFP-specific antibodies showed progressively increasing transgene expression in intracerebral tumor tissues harvested at 2- and 3-week time points after injection of ACE-GFP (Fig. 3A–C). Through FACS analysis of freshly dissected gliomas performed as described above, the percentage of GFP-positive cells was determined to be 74 and 98.1%, respectively, at 2- and 3-week time points after injection of ACE-GFP (Fig. 3D–F).
FIG. 3.
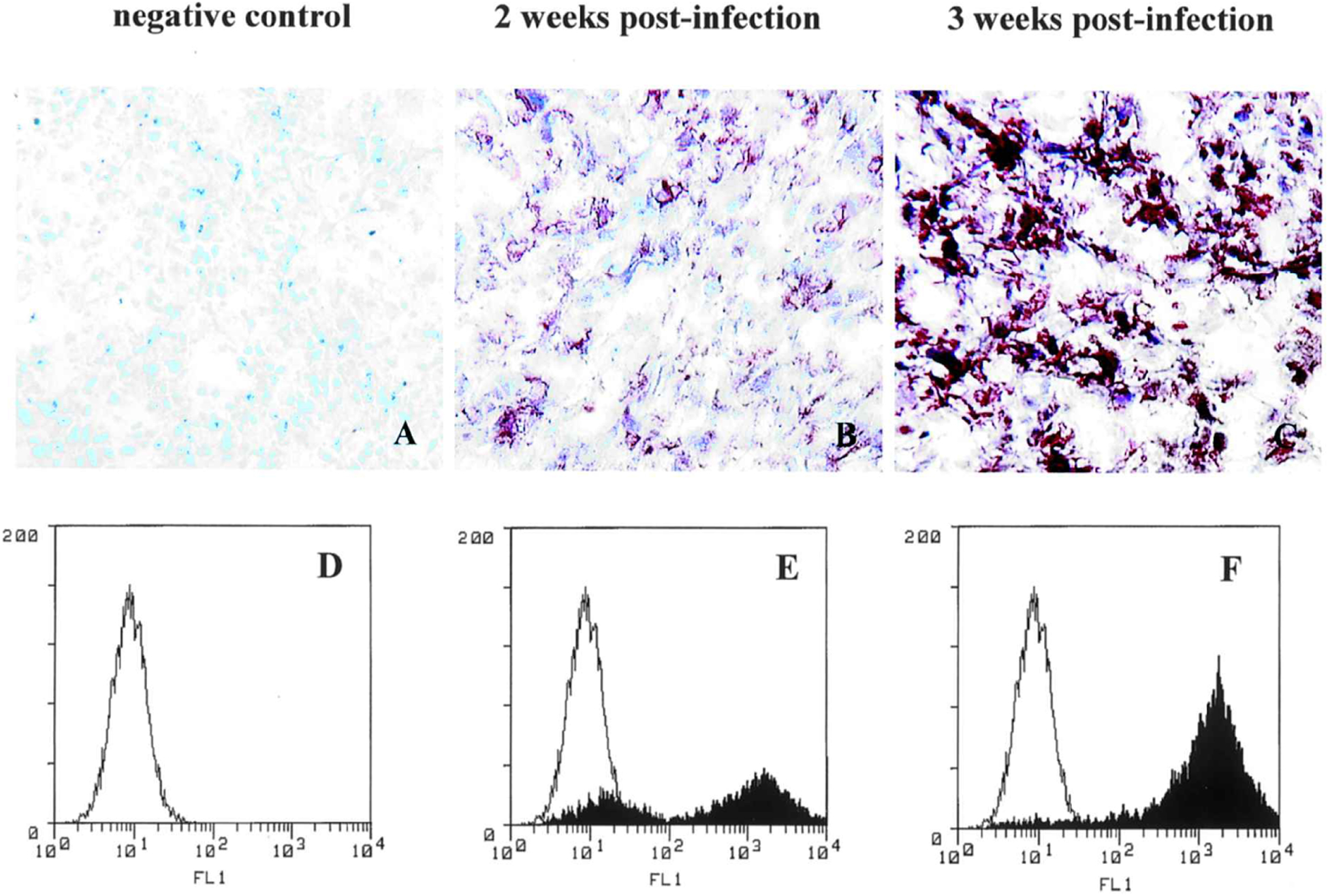
Intracranial U-87 gliomas in athymic nu/nu mice are efficiently transduced by ACE-GFP. (A–C) Immunohistochemistry was performed to stain for GFP expression after sacrifice at weekly intervals after ACE-GFP inoculation. (A) Untransduced U-87 tumor injected with PBS vehicle only, negative control; (B) U-87 tumor, 2 weeks postinjection with ACE-GFP (1.2 × 104 TU/10 ml); (C) U-87 tumor, 3 weeks postinjection with ACE-GFP. (D–F) Single-parameter FACS histograms of freshly dissected tumor cells to quantitate transduction efficiency: (D) Negative control U-87 tumor injected with PBS; (E) U-87 tumor, 2 weeks postinjection with ACE-GFP, 74% positive; (F) U-87 tumor, 3 weeks postinjection with ACE-GFP, 98.1% positive.
No detectable transduction by ACE-GFP in extratumoral and extracerebral organs
A sensitive PCR assay was applied to confirm the presence of stably integrated ACE-GFP sequences in genomic DNA from the brain tumor, and to examine the biodistribution of the RCR vector in peritumoral normal brain as well as extracranial tissues and organs, including lung, liver, kidney, upper GI tract, lower GI tract, skin, spleen, and bone marrow. All these tissues were harvested from nude mice bearing intracranial gliomas at the last time point (3 weeks after vector injection), at which point GFP expression in the tumor was quantified as 98.1% positive by FACS. All genomic DNA samples were processed in parallel and amplified by PCR using primers specific for the GFP transgene sequence; we have previously found that this procedure is sensitive enough to detect as few as 35 copies of the provirus sequence in 0.5-μg genomic DNA samples (Logg et al., 2001b). A 525-bp fragment of the mouse β-casein gene was also amplified as an internal control for the PCR procedure. The results demonstrate that ACE-GFP could not be detected by this sensitive PCR assay in peritumoral normal brain or in the systemic organs even at late time points, although as expected, the RCR vector could be readily detected in the transduced brain tumor (Fig. 4).
FIG. 4.
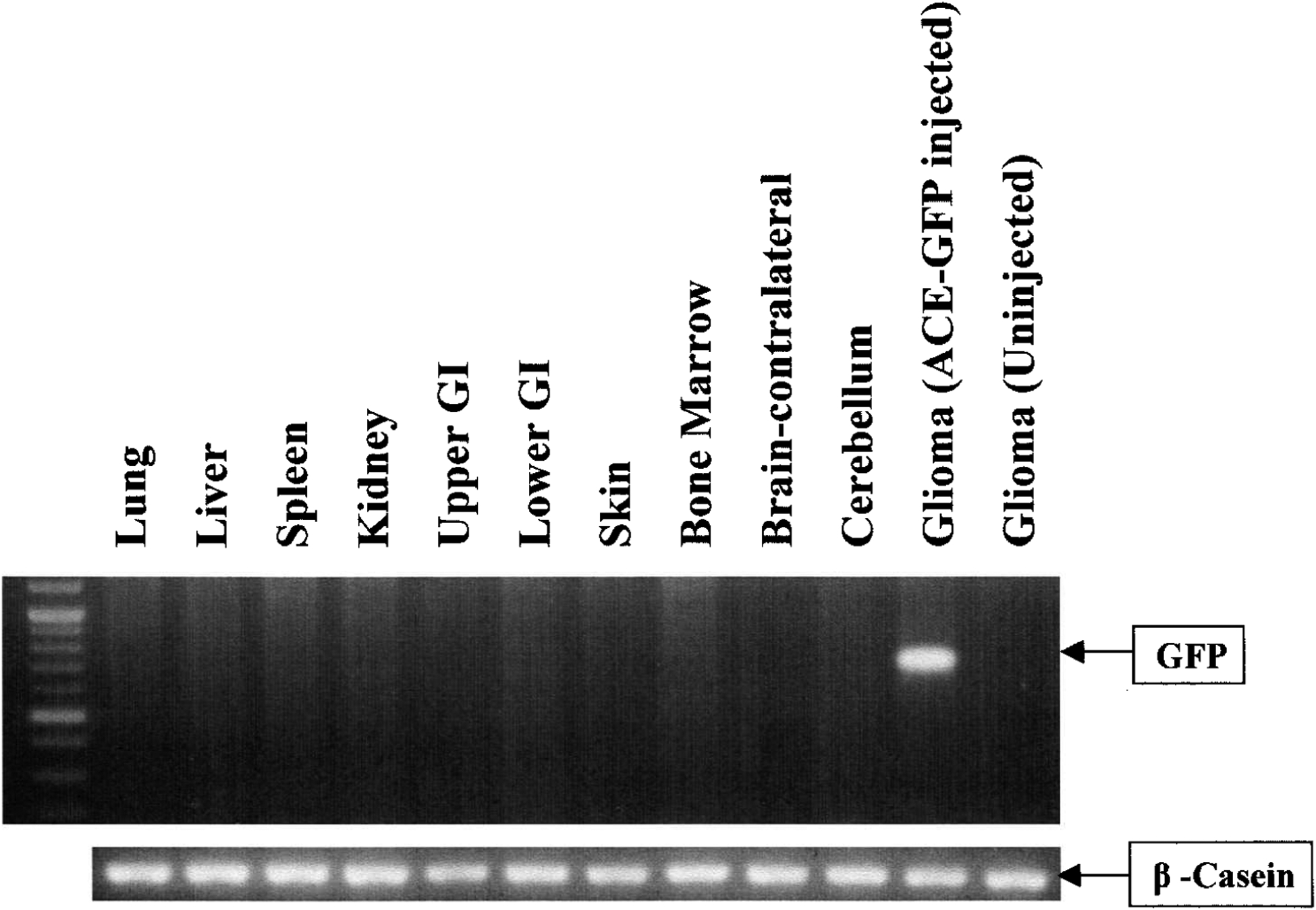
RCR biodistribution after intratumoral injection, as determined by genomic PCR analysis. Genomic DNA (0.5 μg) from athymic nu/nu mice with intracranial gliomas injected with ACE-GFP was isolated from U-87 glioma, contralateral supra-tentorial hemisphere (normal brain), bone marrow, skin, lung, liver, spleen, kidney, upper GI tract (esophagus and stomach), and lower GI tract (colon and small intestine) and was analyzed by PCR. The expected size of the full-length GFP product is 700 bp. Only the injected transduced tumor shows a detectable GFP signal. β-Casein gene, a 525-bp fragment, was also amplified as an internal control. Uninjected glioma was used as a negative control.
ACE-CD significantly improves survival of athymic mice implanted with U-87 intracerebral gliomas after treatment with 5-FC
To determine whether the high level of transduction efficiency achieved by replicating retrovirus vectors could lead to therapeutic benefit, we then constructed and tested an RCR vector expressing the yeast cytosine deaminase suicide gene (ACE-CD; Fig. 1A), and tested this vector in the intracranial U-87 glioma model. One week after tumor implantation, approximately 1.0 × 104 TU in a total volume of 10 μl was stereotactically injected into intracranial U-87 tumors in 2 groups of mice (n = 9 each). An additional group (n = 9) received only PBS vehicle control. Eight days after vector transduction, the prodrug 5-fluorocytosine (5-FC), 500 mg/kg per day, was given for 15 consecutive days by daily intraperitoneal injection to one of the ACE-CD-injected groups and to the PBS vehicle-injected group. The remaining ACE-CD-injected group received only daily intraperitoneal injections of PBS for a consecutive 15 days. The mice treated with ACE-CD plus 5-FC prodrug showed complete survival for a follow-up period of more than 60 days (Fig. 5), compared with mice treated with either ACE-CD/PBS (p < 0.0001) or PBS/5-FC prodrug (p < 0.0001).
FIG. 5.
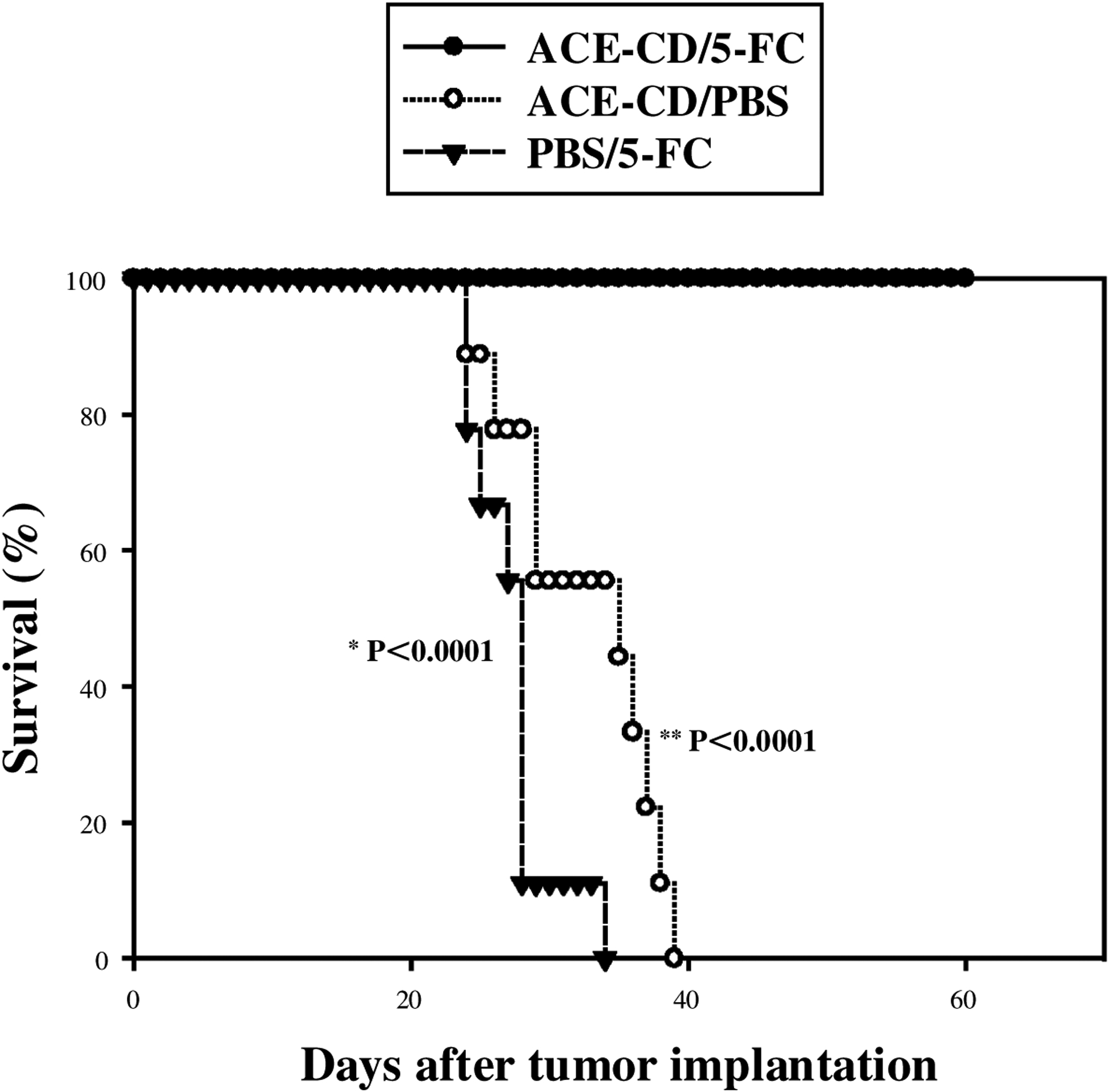
Kaplan–Meier curve demonstrating survival of athymic nu/nu mice injected intracerebrally with U-87 glioma cells. Survival curves were constructed for the three groups: (1) treatment with ACE-CD plus 5-FC prodrug, (2) ACE-CD with PBS instead of prodrug, and (3) PBS injection, followed by 5-FC (see text for details). *ACE-CD/5-FC versus PBS/5-FC; **ACE-CD/5-FC versus ACE-CD/PBS.
DISCUSSION
The results of our present study demonstrate that RCR vectors can efficiently transduce and stably propagate in malignant human glioma cell lines over multiple infection cycles, thereby achieving a tremendous in situ amplification effect after initial administration of a small inoculum in vitro. Furthermore, as predicted from their robust replicative capabilities, intratumoral injection of RCR vector supernatants achieved at least 100-fold higher efficiency compared with injection of conventional replication-defective retrovirus vectors at the same dosage. As expected, the latter showed poor transduction efficiencies of less than 1%, consistent with the results observed in clinical trials (Rainov, 2000). Thus, RCR vectors were found to be capable of spreading and transmitting an inserted transgene throughout entire subcutaneous and intracranial glioma masses in vivo.
Of course, uncontrolled spread of replication-competent retrovirus has the potential to result in insertional mutagenesis and carcinogenesis, as witnessed by the development of lymphomas in 3 of 10 rhesus macaques on transplantation of bone marrow cells heavily contaminated with RCR (Donahue et al., 1992). Although similar experiments using less immunocompromised hosts resulted in no evidence of RCR-induced pathology (Cornetta et al., 1990, 1991), the potential for such adverse events has long been a primary concern of investigators, and may now even have a clinical precedent (Marshall, 2002). However, in contrast to gene replacement therapy, a number of considerations mitigate this concern when contemplating the use of RCR vectors expressing suicide genes for application as a cancer therapeutic agent.
First, because of their absolute requirement for cell mitosis in order to achieve successful infection, we observed that MuLV-based vectors showed an inherent tumor selectivity, only transducing actively dividing glioma cells, but not quiescent normal cells, even when injected directly into normal brain parenchyma. In fact, although ACE-GFP was detected in actively proliferating tumor cells, there was no detectable GFP in peritumoral normal brain or in the contralateral brain hemisphere. The possibility that the RCR vectors might transduce and possibly mutagenize noncancerous, activated replicating cells within or immediately surrounding the tumor, such as neovascular endothelial cells, tumor infiltrating lymphocytes, and peritumoral astrocytes, still requires more careful examination in future experiments; even in this case, however, the incorporation of suicide genes into the RCR vector will ensure that these inadvertantly transduced cells will have a high probability of eventually being eliminated. Although the possibility also exists that the RCR vector might be transmitted to extracranial tissues, particularly tissues containing a higher percentage of mitotic cells, we were not able to detect the vector by PCR analysis in any systemic organ or tissue examined, including bone marrow, spleen, skin, and GI tract. Nonetheless, it is certainly conceivable that hematogenous spread could result in low levels of systemic RCR dissemination, which might remain below the detection limit of our assay due to the relatively short period of analysis afforded by murine models.
The ability of RCR vectors to selectively replicate in dividing glioma cells enables several potential strategies for vector administration in a clinical setting. In small gliomas or gliomas located in surgically inaccessible regions of the brain, RCR vectors may be stereotactically injected into the tumor itself. In large gliomas that may be surgically debulked, RCR may be injected into the resected tumor cavity. Moreover, the risk of eliciting an encephalitic immune response or inadvertently transducing normal quiescent astrocytes or neurons would be considerably less compared with adenovirus- and herpesvirus-mediated gene transfer strategies. In fact, even replication-defective adenovirus vectors have been associated with the development of an immune response leading to tissue damage and destruction of vector-transduced cells, leading to gliosis and encephalopathy in animal models (Dewey et al., 1999), and the use of replication-competent adenovirus vectors for oncolytic therapy may prove even more problematic. Similarly, herpes simplex virus is naturally neurotropic and even attenuated replication-competent strains have the potential for reversion to a more virulent phenotype that could cause severe encephalitis, whereas replication-defective vectors have been so severely attenuated that their overall efficacy is often limited in scope (Martuza et al., 1991).
Although the capacity of our current RCR vector limits the size of the expression cassettes that may be inserted at the env-3’ UTR boundary to approximately 1.3 kb, we were able to insert the cDNA encoding yeast cytosine deaminase as a suicide transgene and tested the ability of the resultant RCR vectors to achieve a therapeutic effect in intracranial glioma models. Because of the aggressive and rapidly fatal time course of the U-87 glioma model when intracranial tumors are established, the RCR vector ACE-CD was injected only 1 week after tumor inoculation. Subsequent administration of the prodrug 5-FC 8 days later resulted in a highly significant survival advantage (p < 0.0001), with 100% survival of the ACE-CD/5-FC-treated animals for at least 60 days, in contrast to animals treated with vector alone or prodrug alone.
We are currently continuing our analysis of these animals, as well as repeating this study with variations in the experimental protocol to determine how parameters such as vector dosage, timing of vector administration, and kinetics of vector spread versus tumor size in vivo might affect the therapeutic efficacy of this approach. RCR vectors are nonlytic and hence replicate without causing immediate cellular damage, and accordingly the induction of immune responses that result in viral clearance might be delayed or attenuated compared with inherently cytolytic viruses such as adenovirus, in which case the nonlytically replicating RCR vectors may ultimately prove better at achieving high levels of intratumoral gene transfer; once adequate levels of transduction are achieved, simultaneous killing of all infected tumor cells can be triggered by administration of the prodrug. Furthermore, the incorporation of a suicide gene into the nonlytic RCR vector itself constitutes a built-in safeguard, as even noncancerous cells infected by the virus would eventually be eliminated by treatment, and the spread of the vector would be inherently self-limited. The transduction efficiency of RCR vectors may be particularly limited in areas of necrosis or hypoxia in malignant gliomas (McLendon et al., 1998); on the other hand, the use of suicide genes that exhibit potent bystander effects should help to offset this potential problem.
Additional levels of selectivity and safety may also be achieved by vector targeting, thus restricting virus replication exclusively to tumor cells, and thereby minimizing the risk to recipients. One approach would be to redirect retroviral vector tropism by modification of the viral envelope protein, thus targeting entry of vectors to cells expressing specific cell membrane proteins (Russell et al., 1993; Kasahara et al., 1994; Valsesia-Wittmann et al., 1994; Snitkovsky and Young, 1998). Redirection of retroviral tropism may also be performed by replacing the retroviral promoter/enhancer with cell-specific transcriptional elements to target viral gene expression to particular tissues. For brain tumors, such transcriptional targeting strategies may involve the use of a glioma-specific promoter such as that of the gene encoding glial fibrillary acidic protein (GFAP), or a radiation-inducible promoter such as that of the gene encoding early growth response protein (EGR), to regulate replication of RCR vectors (Ohga et al., 1996). The development of glioma-specific targeting strategies would further enhance the usefulness of RCR vectors as a therapeutic modality for malignant human brain tumors.
ACKNOWLEDGMENTS
The authors thank Dr. Christopher Logg, Dr. Paula Cannon, and Dr. W. French Anderson for many helpful suggestions and discussion. The authors also thank Dr. Pradip Roy-Burman for generously providing the yeast cytosine deaminase cDNA, and the USC Flow Cytometry Core Facility for assistance with FACS analyses. This work was supported in part by awards from the Connell Foundation (T.C.C.) and the Kriegel Foundation (T.C.C.), and by NIH grant P01 CA59318-07 (N.K.).
REFERENCES
- CANNON PM, KIM N, KINGSMAN SM, and KINGSMAN AJ (1996). Murine leukemia virus-based Tat-inducible long terminal repeat replacement vectors: A new system for anti-human immunodeficiency virus gene therapy. J. Virol 70, 8234–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN TC, HINTON DR, and APUZZO MLJ (1995). Malignant progression in gliomas. In Benign Cerebral Gliomas, Vol. 1. Apuzzo MLJ, ed. (American Association of Neurological Surgeons, Park Ridge, IL: ) pp. 181–189. [Google Scholar]
- CORNETTA K, MOEN RC, CULVER K, MORGAN RA, MCLACHLIN JR, STURM S, SELEGUE J, LONDON W, BLAESE RM, and ANDERSON WF (1990). Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum. Gene Ther 1, 15–30. [DOI] [PubMed] [Google Scholar]
- CORNETTA K, MORGAN RA, GILLIO A, STURM S, BALTRUCKI L, O’REILLY R, and ANDERSON WF (1991). No retroviremia or pathology in long-term follow-up of monkeys exposed to a murine amphotropic retrovirus. Hum. Gene Ther 2, 215–219. [DOI] [PubMed] [Google Scholar]
- COULOMBE J, AVIS Y, and GRAY DA (1996). A replication-competent promoter-trap retrovirus. J. Virol 70, 6810–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY RA, MORRISSEY G, COWSILL CM, STONE D, BOLOGNANI F, DODD NJ, SOUTHGATE TD, KLATZ-MANN D, LASSMANN H, CASTRO MG, and LOWEN-STEIN PR (1999). Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: Implications for clinical trials. Nat. Med 5, 1256–1263. [DOI] [PubMed] [Google Scholar]
- DONAHUE RE, KESSLER SW, BODINE D, MCDONAGH K, DUNBAR C, GOODMAN S, AGRICOLA B, BYRNE E, RAFFELD M, MOEN R, BACHER J, ZSEBO KM, and NIENHUIS AW (1992). Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med 176, 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBRIDGE RB, TANG P, HSIA HC, PHAIK-MOOI L, MILLER JH, and CALOS MP (1987). Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol 7, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASAHARA N, DOZY AM, and KAN YW (1994). Tissue-specific targeting of retroviral vectors through ligand–receptor interactions. Science 266, 1373–1376. [DOI] [PubMed] [Google Scholar]
- KRUSE CA, ROPER MD, KLEINSCHMIDT-DEMASTERS BK, BANUELOS SJ, SMILEY WR, ROBBINS JM, and BURROWS FJ (1997). Purified herpes simplex thymidine kinase retrovector particles. I. In vitro characterization, in situ transduction efficiency, and histopathological analyses of gene therapy-treated brain tumors. Cancer Gene Ther. 4, 118–128. [PubMed] [Google Scholar]
- LEWEKE F, DAMIAN MS, SCHINDLER C, and SCHACHEN-MAYR W (1998). Multidrug resistance in glioblastoma: Chemosensitivity testing and immunohistochemical demonstration of P-glycoprotein. Pathol. Res. Pract 194, 149–155. [DOI] [PubMed] [Google Scholar]
- LOGG CR, LOGG A, TAI CK, CANNON PM, and KASAHARA N (2001a). Genomic stability of murine leukemia viruses containing insertions at the Env-39 untranslated region boundary. J. Virol 75, 6989–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOGG CR, TAI CK, LOGG A, ANDERSON WF, and KASAHARA N (2001b). A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum. Gene Ther 12, 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA HI, GUO P, LI J, LIN SZ, CHIANG YH, XIAO X, and CHENG SY (2002). Suppression of intracranial human glioma growth after intramuscular administration of an adeno-associated viral vector expressing angiostatin. Cancer Res. 62, 756–763. [PubMed] [Google Scholar]
- MANDL S, HIX L, and ANDINO R (2001). Preexisting immunity to poliovirus does not impair the efficacy of recombinant poliovirus vaccine vectors. J. Virol 75, 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL E (2002). Clinical research: Gene therapy a suspect in leukemia-like disease. Science 298, 34–35. [DOI] [PubMed] [Google Scholar]
- MARTUZA RL, MALICK A, MARKERT JM, RUFFNER KL, and COEN DM (1991). Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252, 854–856. [DOI] [PubMed] [Google Scholar]
- MCLENDON RE, ENTERLINE DS, TIEN RD, THORSTAD WL, and BRUNER JM (1998). Tumors of central neuroepithelial origin. In Russell & Rubinstein’s Pathology of Tumors of the Nervous System, 6th Ed. Bigner DD, McLendon RE, and Bruner JM eds. (Arnold, London: ) pp. 307–573. [Google Scholar]
- NANDA D, VOGELS R, HAVENGA M, AVEZAAT CJ, BOUT A, and SMITT PS (2001). Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 61, 8743–8750. [PubMed] [Google Scholar]
- OHGA T, KOIKE K, ONO M, MAKINO Y, ITAGAKI Y, TANIMOTO M, KUWANO M, and KOHNO K (1996). Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 56, 4224–4228. [PubMed] [Google Scholar]
- OSMAK M, VRHOVEC I, and SKRK J (1999). Cisplatin resistant glioblastoma cells may have increased concentration of urokinase plasminogen activator and plasminogen activator inhibitor type 1. J. Neurooncol 42, 95–102. [DOI] [PubMed] [Google Scholar]
- RAINOV NG (2000). A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther 11, 2389–2401. [DOI] [PubMed] [Google Scholar]
- REIK W, WEIHER H, and JAENISCH R (1985). Replication-competent Moloney murine leukemia virus carrying a bacterial suppressor tRNA gene: Selective cloning of proviral and flanking host sequences. Proc. Natl. Acad. Sci. U.S.A 82, 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL SJ, HAWKINS RE, and WINTER G (1993). Retroviral vectors displaying functional antibody fragments. Nucleic Acids Res. 21, 1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHU HK, KIM MM, CHEN P, FURMAN F, JULIN CM, and ISRAEL MA (1998). The intrinsic radioresistance of glioblastoma-derived cell lines is associated with a failure of p53 to induce p21BAX expression. Proc. Natl. Acad. Sci. U.S.A 95, 14453–14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILEY WR, LAUBERT B, HOWARD BD, IBANEZ C, FONG TC, SUMMERS WS, and BURROWS FJ (1997). Establishment of parameters for optimal transduction efficiency and antitumor effects with purified high-titer HSV-TK retroviral vector in established solid tumors. Hum. Gene Ther 8, 965–977. [DOI] [PubMed] [Google Scholar]
- SNITKOVSKY S, and YOUNG JA (1998). Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. U.S.A 95, 7063–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUHLMANN H, JAENISCH R, and MULLIGAN RC (1989). Construction and properties of replication-competent murine retroviral vectors encoding methotrexate resistance. Mol. Cell. Biol 9, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGHIAN A, DUBOIS W, BUDACH W, BAUMANN M, FREE-MAN J, and SUIT H (1995). In vivo radiation sensitivity of glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys 32, 99–104. [DOI] [PubMed] [Google Scholar]
- VALSESIA-WITTMANN S, DRYNDA A, DELEAGE G, AUMAILLEY M, HEARD JM, DANOS O, VERDIER G, and COSSET FL (1994). Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. J. Virol 68, 4609–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARELA-ECHAVARRIA A, PROROCK CM, RON Y, and DOUGHERTY JP (1993). High rate of genetic rearrangement during replication of a Moloney murine leukemia virus-based vector. J. Virol 67, 6357–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGHESE S, NEWSOME JT, RABKIN SD, MCGEAGH K, MAHONEY D, NIELSEN P, TODO T, and MARTUZA RL (2001). Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum. Gene Ther 12, 999–1010. [DOI] [PubMed] [Google Scholar]


