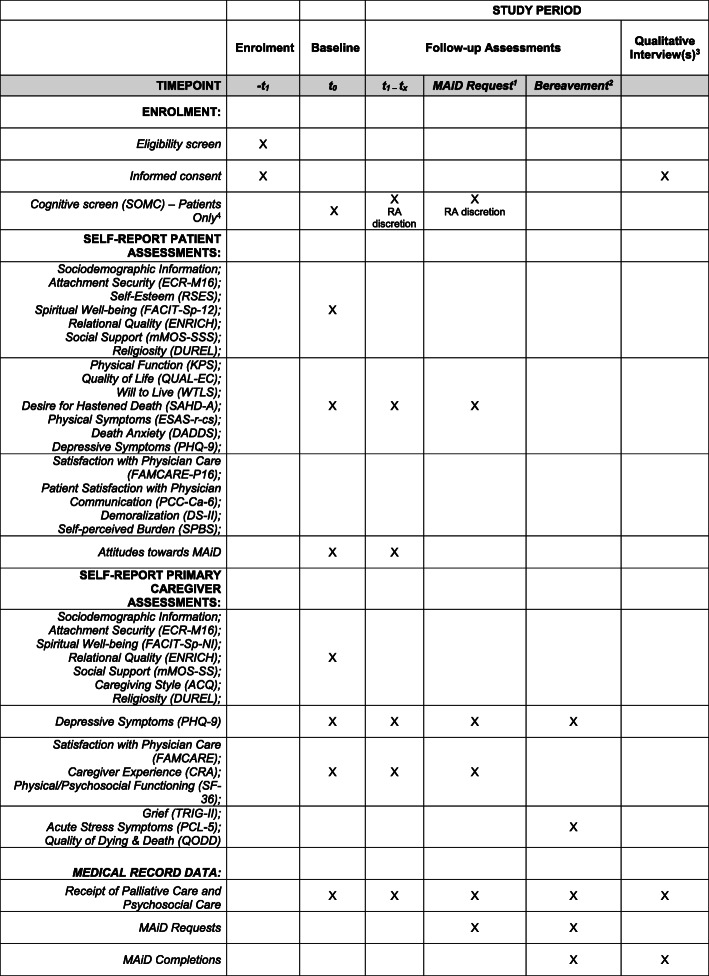
Fig. 2.
1If a patient requests MAiD during the study, even if it falls between the 6 monthly scheduled time points, the patient and their primary caregiver (if applicable), will be invited to complete a follow-up assessment and a qualitative interview at this time. 2In the event that a patient dies during the study (whether by MAiD or other causes), the patient’s primary caregiver (if applicable), will be approached within 6 months of the patient’s death to complete a final follow-up bereavement assessment and a qualitative interview. 3In addition to qualitative interviews with patients requesting MAiD and their participating caregivers, and bereavement interviews with participating caregivers whose loved ones die during the study, a subset of participants (patients and caregivers) will be identified through purposeful sampling and invited to complete one or more qualitative interview(s) at the 6 monthly follow-up time points over the course of the study. 4The SOMC may be re-administered at the discretion of the study research assistant if they feel the patient’s cognitive status may be impaired or have declined since the previous assessment. In the event that a patient fails a cognitive screen during the study, they will be withdrawn by the study principal investigator