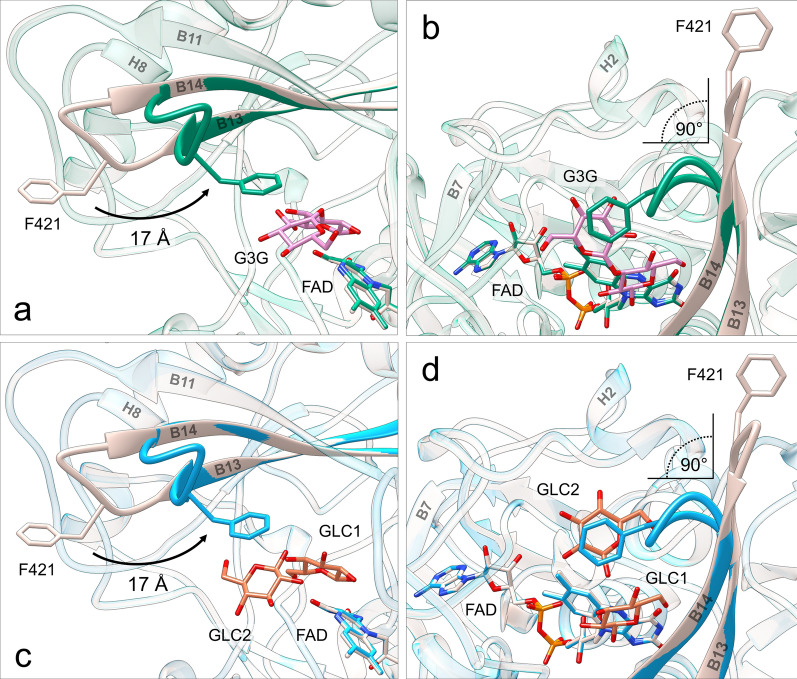
Fig. 5.
Conformational rearrangement of the substrate-binding loop upon sugar binding. Superposition of ligand-free ODH (gray) and ODH-G3G (green): a side view (perpendicular to the loop hinge axis), b top view (along the loop hinge axis). Superposition of ligand-free ODH (gray) and ODH-GLC (light blue) structures: c side view, d top view. G3G and GLC C atoms are in pink and orange, respectively, O atoms in red and N atoms in blue. For clarity sake, only β anomers are represented. Phe421 (also shown in sticks) is exposed to the bulk in the ligand-free structure and shifts ~ 17 Å toward the active site upon binding of sugars, establishing CH-π interactions with the non-reducing glucosyl unit of G3G, or with GLC2 in ODH-GLC. This movement causes the substrate-binding loop to rotate by ~ 90° and wrap toward the active site