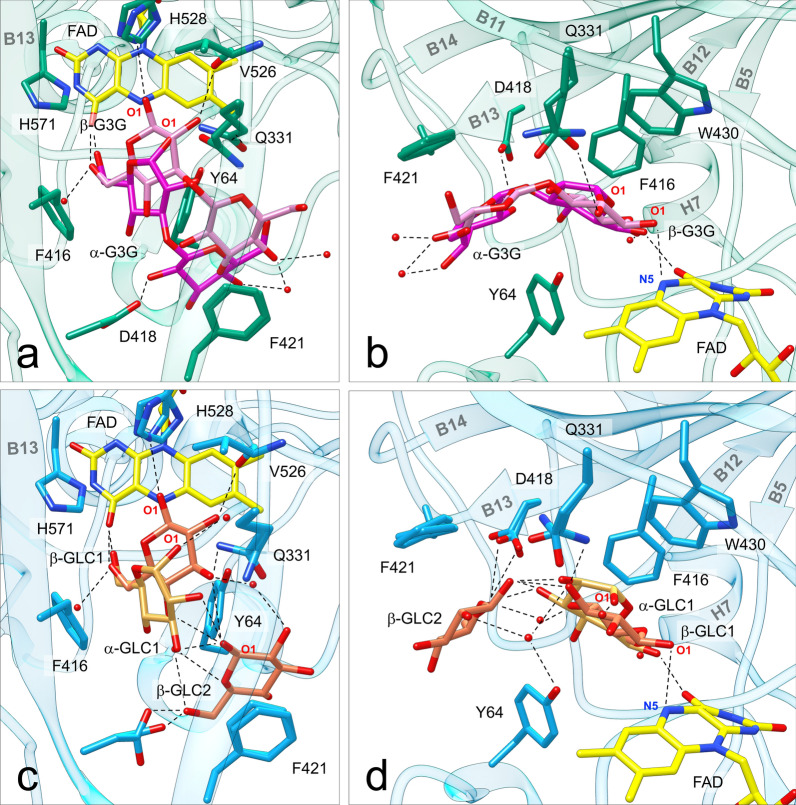
Fig. 6.
Sugar substrates bound to ODH active site. a Top view (perpendicular to the isoalloxazine plane) and b side view of ODH-G3G: the protein is shown in green, the FAD cofactor in yellow, G3G in pink (β anomer) or magenta (α anomer). c Top view and d side view of ODH-GLC: the protein is shown in light blue, the FAD cofactor in yellow, GLC in orange (β anomer) or gold (α anomer); O atoms are in red, N atoms in blue. In both structures the β anomer is closer to the FAD cofactor than the α anomer, and oriented with hydrogen bonds between the reactive O1 atom and His528 and the FAD N5 atom. Both sugars are sandwiched on top of Tyr64 by the aromatic residues Phe421, Phe416 and Trp430. Water molecules are depicted as red spheres. Hydrogen bonds (distance < 3.2 Å) are represented with dotted lines