Abstract
A 49-year-old woman presented to the hospital with shortness of breath 2 weeks after a left total hip replacement. She was found to have a submassive pulmonary embolism (PE), with her case complicated by the detection of a large mobile clot in transit extending through a patent foramen ovale between the right and left atria. The presence of this free-floating right heart thrombus (FFRHT) increases her risks of stroke and mortality, yet the optimal approach to her treatment was unclear. Ultimately, intravenous tissue plasminogen activator was administered with resolution of the clot. Treatment was complicated by haemodynamically insignificant bleeding at the site of recent surgery. Herein, we further discuss the implications and treatment options for patients with an FFRHT in the setting of an acute PE.
Keywords: venous thromboembolism, intensive care, medical management, pulmonary embolism
Background
The presence of a free-floating right heart thrombus (FFRHT) is a dangerous and rare phenomenon that can occur in association with acute pulmonary embolism (PE). Echocardiographic studies have estimated the incidence of FFRHT to be 7%–18% in patients with PE.1 FFRHTs are thought to represent emboli that have traveled from the deep veins of the extremities to the right heart (RH), and can further embolise and lead to a potentially fatal PE or stroke in the presence of a patent foramen ovale (PFO). In addition, FFRHTs have been associated with increased right ventricular (RV) dysfunction, hypotension and significantly higher 30-day mortality rates than in patients with acute PE without FFRHTs.2 3 Studies have shown that mortality rates can be as high as 44.7%, irrespective of therapeutic option used.4
The presence of a FFRHT requires emergency evaluation due to high risk of imminent and often fatal massive PE. However, the optimal approach to treatment remains unclear. Prospective and retrospective cohort studies to date have either failed to show significant differences in mortality between treatment approaches, or differed in their recommendations for treatment.4
Case presentation
The patient is a 49-year-old woman with a medical history of Hodgkin lymphoma treated with chemotherapy and allogenic bone marrow transplant, complicated by graft-versus-host disease as well as avascular necrosis of bilateral hips requiring left total hip replacement. She was discharged home post operatively on aspirin 325 mg two times per day, ambulating with a walker and participating in daily physical therapy.
Two weeks later, she presented to the emergency department with a 1-day history of shortness of breath associated with left calf pain. On presentation, she was afebrile with heart rate 110 beats per minute, respiratory rate 20 breaths per minute, blood pressure 100/70 mm Hg and oxygen saturation of 100% on 2 L/min of oxygen by nasal cannula. Physical examination was unremarkable, and without evidence of RV failure.
Investigations
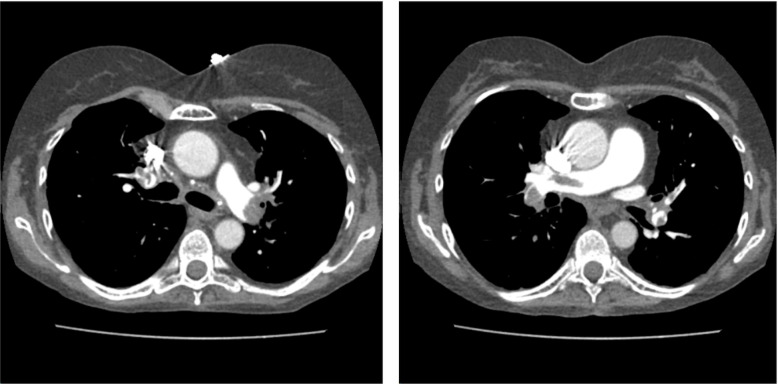
Labs were notable for troponin of 0.3 ng/mL (normal value <0.3 ng/mL) and pro-brain natriuretic peptide (BNP) of 8767 pg/mL (normal value <100 pg/mL). ECG showed sinus tachycardia with new T wave inversions in leads V1–3. CT pulmonary artery angiogram showed bilateral large central PE with evidence of RH strain (figure 1). Transthoracic echocardiogram (TTE) showed RV dilation and a large FFRHT in the right atrium (figure 2). Left lower extremity duplex ultrasound revealed acute occlusive deep vein thrombosis of the femoral vein. Two days later, despite starting anticoagulation, repeat TTE demonstrated extension of the RA thrombus across the interatrial septum with a mobile component in the left atrium (figure 3).
Figure 1.
CT angiography demonstrating bilateral large central pulmonary emboli.
Figure 2.
TTE showing a large linear mobile echo density in the right atrium crossing the tricuspid valve into the right ventricle. TTE, transthoracic echocardiogram.
Figure 3.
TTE showing mobile echodensity (red arrow) in the left atrium concerning for clot-in-transit across a suspected PFO. LA, left atrium; LV, left ventricle; 2D, two dimension; PFO, patent foramen ovale; TTE, transthoracic echocardiogram.
Treatment
The patient was admitted to the medical intensive care unit (ICU) and started on anticoagulation with intravenous unfractionated heparin. Initially intravenous or catheter-directed tissue plasminogen activator (tPA) was deferred given concern for bleeding related to recent surgery. However, due to the extension of the RA thrombus across the interatrial septum with a mobile component in the left atrium, there was now an increased risk for paradoxical embolisation and stroke. Surgical embolectomy and intravenous tPA administration was reconsidered. Given previous mediastinoscopy, surgical embolectomy was deferred. The heparin infusion was held and intravenous tPA was administered in a dose of 10 mg bolus, followed by 90 mg infusion over 1 hour, for a total of 100 mg. Standard stroke dosing was used rather than PE dosing due to high risk of stroke given the FFRHT progressing to the left heart through a PFO.
Outcome and follow-up
Three hours post intervention, repeat TTE showed resolution of the thrombus and improved RV function. Heparin infusion was resumed 2 hours after tPA administration. Overnight, the patient had increased left hip pain coupled with decreased haemoglobin (100 to >84 g/L). CT of the hip was performed revealing large left intramuscular haematoma (12.1×8.7×6.1 cm) (figure 4). The patient remained haemodynamically stable. She was maintained on heparin drip with a lower therapeutic goal (AntiXa: 0.3–0.5 IU/mL). Surveillance CT showed a stable haematoma. The patient never required blood transfusion, was discharged home on anticoagulation and was seen in follow-up without evidence of further bleeding and with resolution of symptoms.
Figure 4.
CT imaging of the left hip demonstrating large haematoma (12.1×8.7×6.1 cm).
Discussion
The presence of FFRHT poses the risk of further embolisation, and in up to 30% of the population, the presence of a PFO can increase the risk of stroke.5 Patients with RV failure from PE are at a higher risk to open PFOs. Treatment options under such circumstances include surgical embolectomy, catheter-directed tPA and systemic tPA. Most studies to date are unable to demonstrate clear survival benefits of one treatment over another. The lack of prospective randomised controlled trials leaves practitioners with little guidance regarding the optimal management of FFRHTs.2 4 6 7
Given the lack of significant and consistent difference in outcomes between the two treatment options, tPA is an appealing first choice given that it is a rapid, and readily available bedside treatment option.4 There have been previous cases of FFRHT treated successfully with systemic tPA demonstrating acceleration of thrombus lysis, pulmonary reperfusion, and improvement of RV function.4 6 One systematic review of 95 studies from 1966 to 2000 suggests that thrombolytic therapy is associated with significantly improved survival rate when compared with surgical embolectomy in patients with an FFRHT.8 This may be attributable to the fact that surgical embolectomy can be delayed pending availability of anaesthesia, operating room, cardiopulmonary bypass and surgical team. On the other hand, tPA, can be obtained and administered quickly at the bedside.
It is important to note that surgical embolectomy is preferred in massive PE, and when thrombolysis is contraindicated. Surgical embolectomy offers the advantage of repairing a PFO simultaneously to reduce the risk of subsequent paradoxical embolism.6 Studies of patients with massive PE treated with surgical embolectomy demonstrate a trend towards lower mortality rate and fewer complications. Also, surgical embolectomy in massive PE results in statistically significant improvements in RV function, pulmonary artery pressures and New York Heart Association (NYHA) score 1 year later as compared with thrombolytic therapy.9 In addition, because of modern surgical advances, some studies suggest that indications for surgical embolectomy should be liberalised to not only patients with massive PE or those who have failed thrombolytic therapy, but also to patients with anatomically extensive or submassive PE.4 The rationale is that early intervention reduces operative risk and that earlier resolution of RV dysfunction leads to less RV failure, infarction and death.4 10
Both systemic tPA and surgical embolectomy are useful treatment options for FFRHT. However, in the absence of haemodynamic instability, one is not better than the other.2 Given the accessibility of tPA, systemic thrombolysis should be strongly considered as a first option, especially when surgical embolectomy cannot be performed. In this case, both surgical embolectomy and systemic tPA were considered, however, due to the patients history of mediastinoscopy, systemic tPA was administered.
The major complication of thrombolytic therapy is significant bleeding, which occurs in as many as 22% of patients.8 Despite the concern for bleeding in the setting of recent hip surgery, the benefit of avoiding an imminent, devastating and possibly fatal stroke outweighed the risk of bleeding.
Given the heterogeneous presentation of patients with PE and FFRHT, and the differences in available resources at each facility, it is important that we continue to use clinical judgement and weigh the benefits and risks of each treatment in every patient’s individual clinical context before deciding on the optimal treatment plan.
Learning points.
When evaluating patients with a free-floating right heart thrombus (FFRHT), it is important to risk stratify patients to see if they are at risk of stroke through echocardiography with injection of agitated saline. The presence of a patent foramen ovale (PFO) and intracardiac shunt increases the risk of stroke, since the FFRHT can more easily move across the PFO to the left atrium.
Thrombolytic therapy can be a safe and effective treatment option for patients with high-risk FFRHT to prevent further embolic events. However, there is a small risk of thrombus fragmentation and increased risk of stroke in the setting of a PFO, therefore, risk versus benefits need to be evaluated.
In patients with submassive pulmonary embolism and FFRHT, surgical embolectomy and thrombolytic therapy are both effective treatment options with similar outcomes.
Footnotes
Contributors: WY performed analysis of data, wrote the paper, obtained patient consent and was involved with editing and reviewing of the paper. IG provided mentorship and was involved with editing and reviewing of the paper. AH collected data, and was involved with the editing and reviewing of this paper. RG was the attending of record and involved in the final editing and reviewing of this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. InMayo Clinic Proceedings, 1984:17–20. [DOI] [PubMed] [Google Scholar]
- 2.Chartier L, Béra J, Delomez M, et al. Free-Floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999;99:2779–83. 10.1161/01.cir.99.21.2779 [DOI] [PubMed] [Google Scholar]
- 3.Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest 2002;121:806–14. 10.1378/chest.121.3.806 [DOI] [PubMed] [Google Scholar]
- 4.Torbicki A, Galié N, Covezzoli A, et al. Right heart thrombi in pulmonary embolism: results from the International cooperative pulmonary embolism registry. J Am Coll Cardiol 2003;41:2245–51. 10.1016/s0735-1097(03)00479-0 [DOI] [PubMed] [Google Scholar]
- 5.Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005;129:1018–23. 10.1016/j.jtcvs.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 6.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:997–1053. 10.5603/KP.2014.0211 [DOI] [PubMed] [Google Scholar]
- 7.Azari A, Beheshti AT, Moravvej Z, et al. Surgical embolectomy versus thrombolytic therapy in the management of acute massive pulmonary embolism: short and long-term prognosis. Heart Lung 2015;44:335–9. 10.1016/j.hrtlng.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 8.Nkoke C, Faucher O, Camus L, et al. Free floating right heart thrombus associated with acute pulmonary embolism: an unsettled therapeutic difficulty. Case Rep Cardiol 2015;2015:1–4. 10.1155/2015/364780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez D, Yusen RD. Clinical significance and management of right heart thrombi: more questions than answers. Eur Respir J 2016;47:702–3. 10.1183/13993003.01968-2015 [DOI] [PubMed] [Google Scholar]
- 10.Mirijello A, D'Errico MM, Curci S, et al. Paradoxical embolism with thrombus stuck in a patent foramen ovale: a review of treatment strategies. Eur Rev Med Pharmacol Sci 2018;22:8885–90. 10.26355/eurrev_201812_16657 [DOI] [PubMed] [Google Scholar]