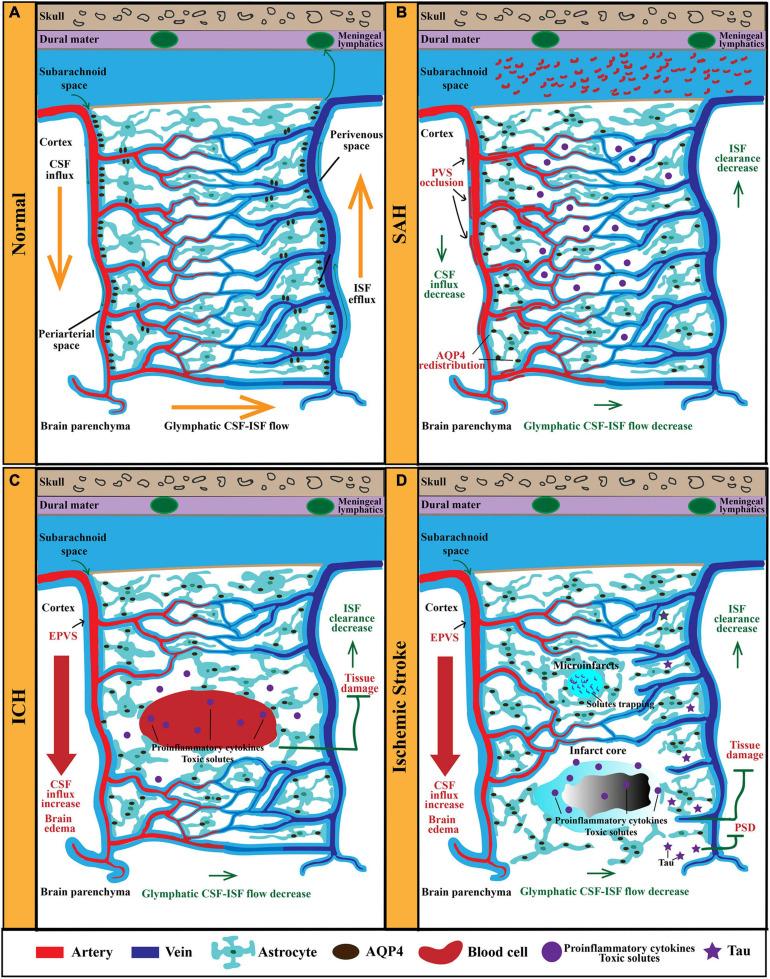
FIGURE 1.
The anatomy and function of the GS in physiological and pathological conditions. (A) The GS mainly consists of periarterial CSF-inflow channel, perivenous ISF-outflow channel, and astrocytes-mediated convective transport of fluid and solutes. AQP4 polarized on astrocytic end-feet facilitates fluid and solutes exchange between the CSF and the brain interstitium. In physiological condition, CSF from the subarachnoid space is propelled into the brain parenchyma via the PVS of penetrating arteries. Then, CSF exchange with ISF in the extracellular space. Afterward, ISF and solutes move toward the perivenous space, ultimately drain out of the CNS via meningeal lymphatics. (B) After SAH, blood components invade the PVS rapidly, resulting in PVS occlusion and reduced CSF influx and ISF clearance. Furthermore, the perivascular polarity of AQP4 decreases after SAH, which resulted in accumulation of proinflammatory cytokines and neurotoxic solutes. In addition, AQP4 in the influx routes is upregulated markedly, while that in the efflux routes changes slightly. (C) In models of ICH, PVS is enlarged and responsible for brain edema. EPVS is also an independent risk factor for ICH recurrence. Moreover, glymphatic clearance rate is reduced, which contributes to the accumulation of proinflammatory cytokines and neurotoxic solutes. (D) In models of ischemic stroke, the ischemic spreading depolarizations along with subsequent vasoconstriction result in EPVS and doubled glymphatic inflow speeds. The increased influx of CSF in the GS contributes to poststroke edema. Additionally, the GS dysfunction after ischemic stroke impedes the clearance of neurotoxic solutes, proinflammation cytokines, and tau, which results in tissue damage and PSD. GS, glymphatic system; CSF, cerebrospinal fluid; ISF, interstitial fluid; PVS, perivascular space; CNS, central nervous system; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; PSD, poststroke dementia.