Dear Editor,
An unusually high prevalence of thromboembolic events has been observed during the course of coronavirus disease 2019 (COVID-19), an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While the mechanisms involved in the hypercoagulable state that can develop into coagulopathy in COVID-19 patients remain to be elucidated, an increased prevalence of anti-phospholipid (aPL) antibodies in COVID-19 patients has been reported by several groups. This is important because the presence of aPL antibodies can result in abnormalities that include prolonged clotting times and/or clinical manifestations that can range from thrombocytopenia to more severe anti-phospholipid syndrome (APS), being the acquired prothrombotic state contributed by aPL antibodies responsible for a significantly increased risk of arterial, venous, and microvascular thrombosis [1].
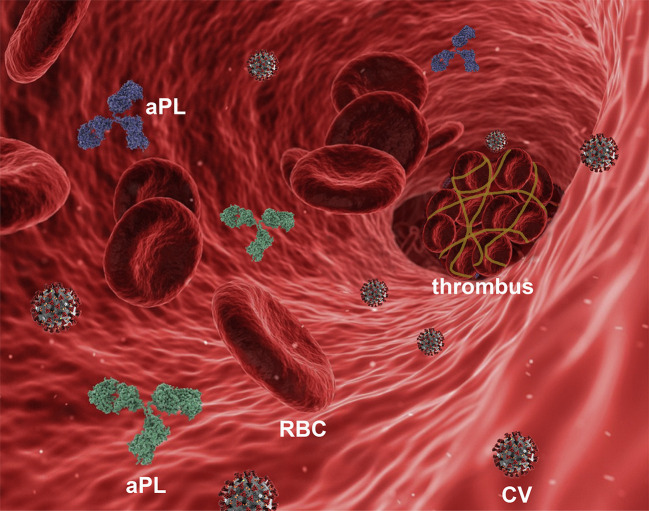
In the case of SARS-CoV-2 infection (Fig. 1 ), early studies identified an unexpected high incidence of aPL antibodies in COVID-19 patients [[2], [3], [4]] in the intensive care unit (ICU) [5], with a cumulative rate of 27.6% within 24 h from the admission to the hospital [6]. Moreover, autopsies revealed deep venous thrombosis in up to 58% of severe COVID-19 patients in which venous thromboembolism had not been previously suspected [7].
Fig. 1.
Schematic representation (not in scale) of a blood vessel with a thrombus and anti-phospholipid antibodies (aPL) in the course of infection with SARS-CoV-2 (CV). RBC, red blood cell.
aPL antibodies typically comprise anti-cardiolipin antibodies, anti-β2-glycoprotein I (β2GPI) antibodies, and lupus anti-coagulants. Less prevalent aPL antibodies include antibodies to prothrombin, phosphatidylserine, phosphatidylethanolamine, and to the phosphatidylserine/prothrombin complex [8].
In a series of 21 ICU hospitalized patients with severe or critical COVID-19, 67% patients had at least a single aPL positivity, 25% had double positivity, and 8% had triple positivity [9]. Anti-cardiolipin IgM were present in 14% of those patients and anti-cardiolipin IgG in 10%. Importantly, 9.5% aPL-seropositive patients died within 30 days after aPL antibody measurements, while 19% remained hospitalized [9].
In another investigation on 31 ICU COVID-19 patients, 7 of 9 thrombotic patients had at least one aPL antibody and 16 of 22 patients without thrombosis were also aPL antibody-positive [10]. aPL autoantibodies were present in 52% of 172 patients hospitalized with COVID-19 and included anti-cardiolipin IgM in 23%, anti-phosphatidylserine/prothrombin (aPS/PT) IgG in 24%, and aPS/PT IgM in 18% [11]. The finding that another study found about 12% prevalence of anti-cardiolipin IgG/M/anti-β2GPI IgG [12] indicates variability among patients' cohorts and sites of measurements.
In non-critically-ill (i.e., non-ICU) COVID-19 patients, aPL antibodies were also common [13], having 47.1% patients at last one positive aPL and double or triple aPL seropositivity being present in 11.1% and 1.9% patients, respectively. Anti-cardiolipin antibodies (mostly of the IgA subtype) were present in 33.7% patients, while anti-β2GPI IgG, IgM and IgA were positive, respectively, in 8.7%, 2.9% and 5.8% of patients. Thrombotic events occurred more frequently in patients with anti-cardiolipin antibodies (45.5% for IgM and IgA; 27.3% for IgG) but high frequency was also observed for anti-β2GPI IgA (27.3%). Although a limitation of that study was the lack of monitoring over time for the persistence of aPL antibodies, the investigation showed nonetheless that aPL antibodies were frequent in non-severely ill hospitalized COVID-19 patients and associated with thrombotic events in 64% cases [13].
aPL antibodies in COVID-19 patients with coagulopathy and infarcts appear mostly represented by anti-cardiolipin IgA antibodies but also IgA and IgG to β2GPI [14]. A meta-analysis found 58% positivity for aPL antibodies in 250 COVID-19 patients, being lupus anti-coagulant present in 64%, anti-cardiolipin in 9%, and anti-β2GPI in 13% patients [15]. Although the positivity for lupus anti-coagulant in COVID-19 patients is generally very high (44.6% to 87%, depending on the studies) and superior to that of anti-cardiolipin IgG/IgM and/or anti-β2GPI antibodies (8.9%), there is no conclusive evidence of a possible correlation between the presence of lupus anti-coagulant and thrombosis in COVID-19 [3,16] although a concomitant presence of lupus-anti-coagulant with anti-cardiolipin IgM or IgG appears strongly associated with thrombosis during acute COVID-19 infection [13].
Of note, studies have suggested that some aPL antibodies may be transient during COVID-19 [10]. In a cohort of COVID-19 patients where 50.6% displayed thrombosis, with 7.5% having at least one recurrence., about half were positive for aPL antibodies, and a strong association was observed between thrombosis and positivity of anti-cardiolipin IgM (41%), also confirmed at follow up of 3–6 months [16].
Interestingly, an elevated frequency of aPL antibodies belonging to the IgA class in severe COVID-19 has led to the suggestion that this finding could underlie the anti-viral response at mucosal (e.g., bronchial) sites [17]. Whether or not this is the case, one cannot neglect that the immune-triggered, complement-mediated thrombotic microangiopathy (TMA) accompanied by cytokine storm, advanced lung inflammation and sepsis that occurs in the disease [18] often associate with widespread thromboses and disseminated intravascular coagulation (DIC), at last in some critically ill COVID-19 patients. In a study on the risk for thrombotic arterial and venous occlusions in COVID-19, the transfer of IgG purified from patients' sera into mice accelerated venous thrombosis in two different mouse models, suggesting a pathogenicity of the circulating antibodies from about half of hospitalized COVID-19 patients [11]. However, other studies did not identify correlations between aPL antibodies and thrombosis [19,20].
At present, the American Society of Hematology suggests caution in interpreting the associations between aPL antibodies and thromboembolic events in COVID-19 and not only because of the different assays used for the detection of aPL antibodies in different centers and the possibility that they can be present transiently. Timing of measurements could be a relevant factor because aPL antibodies seem more frequent in critically ill as compared to non-critically ill COVID-19 patients and emerging about 35–39 days after disease onset [21], being anti-β2GPI IgA the aPL antibodies most common (28.8% of critically ill patients), followed by anti-cardiolipin IgA (25.8%) and anti-β2GPI IgG (18.2%) [20]. Yet the finding that aPL are frequently present in COVID-19 patients (Table I ) poses several questions. For example, are aPL antibodies specifically induced by COVID-19 and, if so, how? How much do aPL antibodies contribute to the prothrombotic state of COVID-19 patients and to the overall clinical picture of the patient? Would all COVID-19 patients with aPL antibodies benefit from early anti-coagulation therapy, even when having different coagulation state characteristics? Addressing these questions will advance the progress in managing COVID-19 and its complications, and possibly lead to an improved therapy of a disease that has rapidly upended routines and working activities for millions of individuals worldwide.
Table I.
Frequency of aPL antibodies in COVID-19 patients from different cohorts.
Funding
None.
Declaration of Competing Interest
None.
References
- 1.Chock Y.P., Moulinet T., Dufrost V., Erkan D., Wahl D., Zuily S. Antiphospholipid antibodies and the risk of thrombocytopenia in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev. 2019;18:102395. doi: 10.1016/j.autrev.2019.102395. [DOI] [PubMed] [Google Scholar]
- 2.Tvito A., Zimmerman F.S., Asher E., Helviz Y. Lupus anticoagulant in patients with COVID-19. Int J Lab Hematol. 2021;43:e17–e18. doi: 10.1111/ijlh.13334. [DOI] [PubMed] [Google Scholar]
- 3.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J Intern Med. 2021;289:422–424. doi: 10.1111/joim.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biggioggero M., Meroni P.L. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev. 2010;9:A299–A304. doi: 10.1016/j.autrev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., Vázquez-Range A., Márquez-Velasco R., Baranda-Tovar F., et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann Rheum Dis. 2020;218100 doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- 10.Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Joncour A., Frere C., Martin-Toutain I., Gougis P., Ghillani-Dalbin P., et al. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun Rev. 2021;20:102729. doi: 10.1016/j.autrev.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gkrouzman E., Barbhaiya M., Erkan D., Lockshin M.D. Reality check on antiphospholipid antibodies in COVID-19–associated coagulopathy. Arthritis Rheumatol. 2021;73:173–180. doi: 10.1002/art.41472. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer O., Tacquard C., Dieudonné Y., Nespola B., Sattler L., Grunebaum L., et al. Follow-up of COVID-19 patients: LA is transient but other aPLs are persistent. Autoimmun Rev. 2021;102822 doi: 10.1016/j.autrev.2021.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali O.H., Bomze D., Risch L., Brugger S.D., Paprotny M., Weber M., et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Z.W., Zhang H.Z., Liu C., Dong K. Autoantibodies in COVID-19: frequency and function. Autoimmun Rev. 2021;20:102754. doi: 10.1016/j.autrev.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11:584241. doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 21.Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., et al. Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheumatol. 2020;72:1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachoyiannopoulos P.G., Magira E., Alexopoulos H., Jahaj E., Theophilopoulou K., Kotanidou A., et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Cao W., Jiang W., Xiao M., Li Y., Tang N., et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50:580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]