The spread of the COVID-19 pandemic has led to unprecedented acceleration of vaccine development. Although tested on thousands of people before regulatory approval, very rare adverse events can manifest during roll-out of vaccines to the general population.
During follow-up of the COVID-19 vaccine roll-out, side-effects related to a hypothetical immune system overstimulation or over-reaction have been described, such as adenoviral vector vaccine-induced immune thrombotic thrombocytopenia1 and systemic immune-mediated diseases after mRNA-vaccine.2, 3 However, to our knowledge, there have been no reports of Adult-onset Still's disease (AOSD) after vaccination with a COVID-19 adenoviral vector vaccine (ie, ChAdOx1 nCoV-19 vaccine).
AOSD is considered a rare inflammatory disorder affecting young adults and IL-1 has a central role in the pathogenesis. Inhibition of IL-1β by monoclonal antibodies and of the IL-1 receptor by antagonists, substantially ameliorates clinical symptoms.4 Increasing evidence points towards a similar role for IL-1 in COVID-19 hyperinflammation, and several reports suggest that inhibition of IL-1 substantially reduces hyperinflammation, respiratory insufficiency, and mortality in patients in hospital with severe COVID-19.5
Here, we report the case of a 36-year-old male, without any comorbidities or family history of autoimmune diseases, who developed AOSD after the first dose of the ChAdOx1 nCoV-19 vaccine. The day after the vaccination, he developed high-grade fever (>39°C) that lasted for 48 h. The fever then returned 4 days later, preceded by sore throat and accompanied by evanescent and not pruritic rash and chest pain. He was treated at home with antibiotics and non-steroidal anti-inflammatory drugs without improvement. He presented to the emergency room of a local hospital (Santo Spirito Hospital, Rome, Italy) due to the increasing severity of his symptoms and was admitted to the internal medicine unit for further exploration. On admission, the patient was febrile with tachycardia, with chest pain exacerbated by inspiration. His pulse was 120 beats per min, temperature was 38·9°C, blood pressure was 120/60 mm Hg, and oxygen saturation was 96%. Nothing relevant was found on physical examination.
After being tested for viral and bacterial agents, which were all negative, given the presence of leukocytosis (30·83 × 109 cells per L with 86·6% neutrophil) and fever, broad-spectrum antibiotic treatment was started, without any clinical or laboratory response during 10 days. The treating physicians suspected that the patient might have an autoimmune condition and so started him on methylprednisolone 0·75 mg/kg, and the fever disappeared after 3 days of treatment. To further investigate the case, the patient was transferred to our rheumatology unit at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy).
21 days after the first episode of fever, physical examination and vital parameters were normal, with chest pain exacerbated by inspiration being an isolated finding. Despite the increase of corticosteroid treatment at 1mg/kg, laboratory tests confirmed persistent leukocytosis, abnormal liver function, high acute-phase reactants (C-reactive protein 188 mg/L, erythrocyte sedimentation rate 85 mm/h, IL-6 11·8 ng/L, soluble IL-2R 9·74 UI/L), high serum ferritin (1482 ng/mL, normal range 12–240 ng/mL), and transitory increased troponin (1695 pg/dL).
RT-PCR SARS-CoV-2 tests were done several times with negative results. Microbiological routine tests (including common viral serology and bacterial blood cultures) were negative for anything clinically relevant. An autoimmune panel was normal, except for the presence of beta-2-glicoprotein IgG (29·7 U/mL) and lupus anticoagulant.
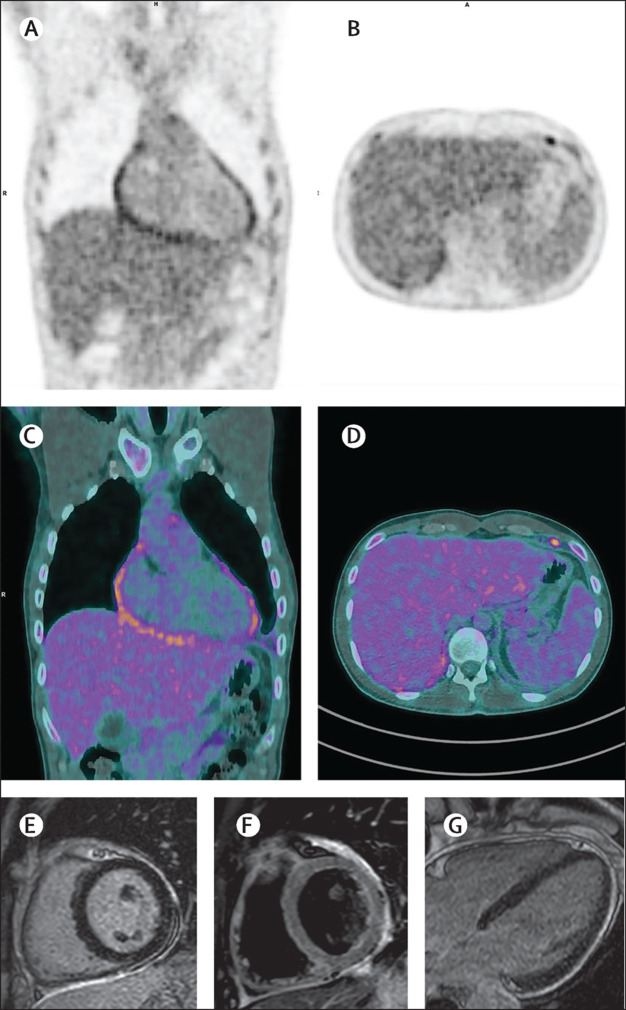
Ecocardiography showed pericardial effusion, without modification of ejection fraction. Lung and abdominal ultrasound assessments and CT scan revealed bilateral pleural effusion, posterior wall pericardial effusion, subcentimetric lymphadenopathy, and mild splenomegaly. A PET-CT scan was done and excluded neoplasms or vasculitis, but confirmed cardiophrenic lymphadenopathies and pleuropericardial effusion. cardiac MRI was compatible with recent myopericarditis (figure ).
Figure.
PET (A, B), PET-CT (C, D), and cardiac MRI (E–G) scans of a patient with recent myopericarditis
The figure shows the involvement of pericardium with three different imaging techniques: PET, PET-CT, and MRI, 14 and 21 days after initial symptoms onset. Images A (coronal PET) and C (fused PET-CT images) show increased radiopharmaceutical uptake in the pericardium, and images B (axial PET) and D (PET-CT slices) in lymph node located in left cardiophrenic angle. MRI delayed-enhancement images acquired on the mid-ventricular short-axis (E) and horizontal long axis (F) planes show very high signal in the pericardium correspondence, almost circumferentially affected. A suspected thin underlying epicardial and myocardial involvement can be seen. On T2-weighted imaging with fat suppression on the same plane as in image E (F) presence of hypo intense components can be seen along the epicardial surface suggesting fibrin deposits.
In accordance with Yamaguchi criteria,4 a diagnosis of AOSD was made and corticosteroid treatment was continued. Because of myocardial involvement, persistent leukocytosis and high ferritin (946 ng/mL), treatment with the IL-1 receptor antagonist anakinra was started (100 mg daily). After 4 days, we observed a complete normalisation of symptoms and blood tests and the patient was discharged.
At 1 month of follow-up, he reported feeling well and ferritin levels and inflammatory markers were within normal limits.
The first case of AOSD after COVID-19 has recently been reported.6 Systemic inflammation, unremitting fever, high serum ferritin, and a hyperinflammatory syndrome that induces major organ involvement characterise both AOSD7 and severe COVID-19,8 suggesting they might be triggered by the same mechanisms.
In the present case, the strong temporal association between the symptoms and COVID-19 vaccination prompted us to suspect a causal connection, since similar AOSD cases have been observed after vaccinations for influenza, hepatitis A, and hepatitis B.9 However, we cannot exclude the possibility that the timing with regards to vaccination was coincidental. Possible pathogenic mechanisms might include high levels of spike protein production after vaccination, that also cause the immune system activation in COVID-19, suggesting that the inflammatory storm of AOSD can be triggered either by the infection or by the vaccination. Intrinsic adjuvant activity of adenoviral particles targeting innate immune cells and ultimately resulting in the production of type I interferon and multiple pro-inflammatory cytokines, might be another potential cause. 10 Finally, the role of vaccines-adjuvants such as polysorbate 80, which is present also in the influenza and hepatitis vaccines, might also be considered.3 The response of COVID-19 to IL-1 blockade, known to produce substantial benefit in AOSD, further supports the hypothesis of a strict pathogenic connection between hyperinflammatory syndromes and severe COVID-19.5
We declare no competing interests. The patient provided informed consent to publish this case.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg J, Thomas A, Iravani A. 18F-fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet. 2021;397:e9. doi: 10.1016/S0140-6736(21)00464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watad A, De Marco G, Mahajna H, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nat Rev Rheumatol. 2018;14:603–618. doi: 10.1038/s41584-018-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamidis AD, Koehler P, di Cristanziano V, et al. First manifestation of adult-onset Still's disease after COVID-19. Lancet Rheumatol. 2021;3:e319–e321. doi: 10.1016/S2665-9913(21)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauter M, Gerfaud-Valentin M, Delplanque M, Georgin-Lavialle S, Sève P, Jamilloux Y. Adult-onset Still's disease complications. Rev Med Interne. 2020;41:168–179. doi: 10.1016/j.revmed.2019.12.003. (in French). [DOI] [PubMed] [Google Scholar]
- 8.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka K, Fujimoto S, Oba H, Minami M, Aoki T. Onset of adult-onset Still's disease following influenza vaccination. Mod Rheumatol. 2011;21:432–435. doi: 10.1007/s10165-011-0418-7. [DOI] [PubMed] [Google Scholar]
- 10.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]