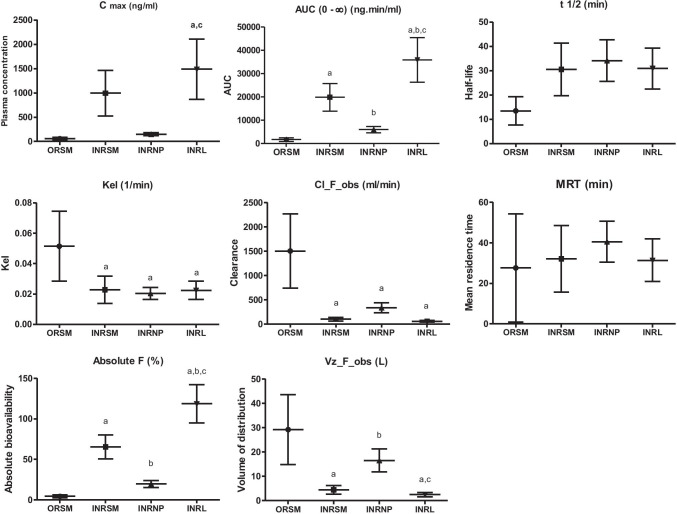
Fig. 4.
Pharmacokinetic features of different rivastigmine formulations. Maximum concentration (Cmax), area under the curve (AUC), elimination rate constant (Kel), half-life (t 1/2), clearance (Cl_F_obs), Mean residence time (MRT), absolute bioavailability (%F), volume of distribution (Vz_F_obs) of rivastigmine, and its formulations in scopolamine-induced rats. Data represented as mean ± SD, ANOVA followed by post-hoc test *a = p < 0.05 vs. ORSM; *b = p < 0.05 vs. INRSM; *c = p < 0.05 vs. INRNP