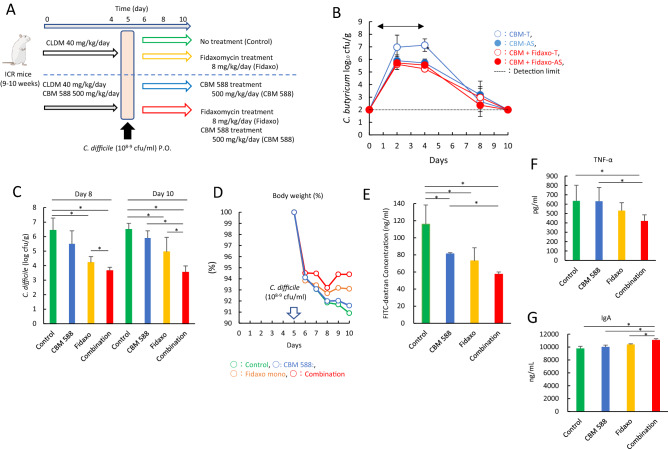
Figure 1.
C. butyricum enhanced C. difficile colonization resistance in the gut and attenuated gut inflammation when used with an anti-C. difficile antibiotic agent. (A) Experimental design of the Clostridioides difficile infection model with 9- to 10-week-old ICR Swiss mice. (B) Enumerating C. butyricum in feces. (open circle): total colony count in CBM 588 administration group (CBM 588-T), (filled circle): spore colony count in CBM 588 monotherapy group (CBM 588-AS), (open circle): total colony count in combination group (Combination-T), (filled circle): spore colony count in combination group (Combination-AS). The testing detectable level is above 2.0 (log amount) per gram of feces, data shown as mean ± SD (n = 5–10 per group). (C) Enumerating C. difficile in feces at day 8 and 10. 1: control group (Control), 2: CBM 588 monotherapy group (CBM 588), 3: fidaxomicin monotherapy group (Fidaxo), and 4: combination (CBM 588 + fidaxomicin) group (Combination). The testing detectable level is above 2.0 (log amount) per gram of feces, data shown as mean ± SD (n = 5–10 per group). (D) Weight changes of control group (green), CBM 588 administration group (blue), fidaxomicin administration group (yellow), and combination group (red) over the duration of the study (from C. difficile inoculation to day 10), data shown as mean (n = 5 per group). (E) Intestinal permeability was determined. All values are mean ± SD (n = 5). (F) Tumor necrosis factor-α (TNF-α) and (G) Immunoglobulin A (IgA) protein concentrations in mice colon tissue detected at day 8. All values are mean ± SD (n = 5–10).