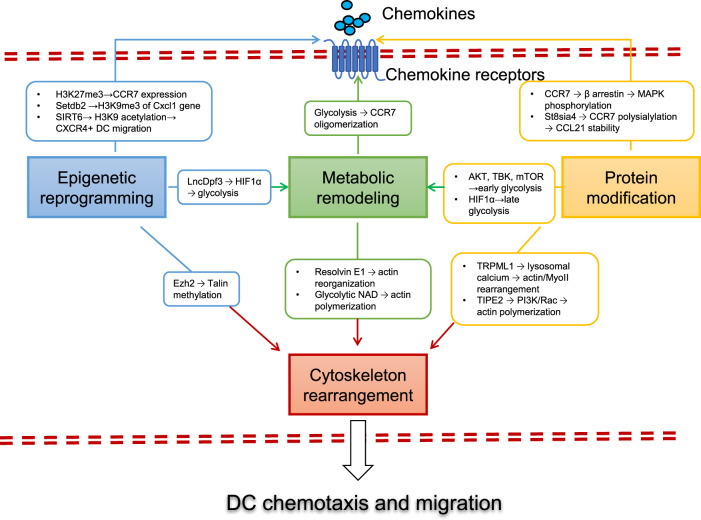
Fig. 2. Molecular regulation of DC migration.
Multiple levels of intracellular regulators are employed to modulate the signaling pathways induced by chemokines to ensure the most beneficial outcome of DC migration in the regulation of immunity and tolerance. (1) Protein modification (shown in yellow boxes and arrows). CCL19 and CCL21 stimulation induces the phosphorylation of CCR7, β-arrestin, MAPK/ERK, p38 and JNK successively, which is crucial for DC chemotaxis and migration. Polysialylation of CCR7 is essential for recognition of its ligand CCL21 and therefore controls CCR7-mediated DC migration. (2) Epigenetic reprogramming (shown in blue boxes and arrows). Migratory cDCs and nonmigratory moDCs show different levels of H3K27me3 modifications at the CCR7 gene locus. Setdb2 induces the generation of H3K9me3 (a repressive marker) at the Cxcl1 gene promoter, leading to reduced neutrophil infiltration and inhibited host defense against bacterial superinfection. SIRT6 promotes CXCR4+ DC migration to the afferent lymph nodes via its effects on monitoring H3K9 acetylation. Ezh2 directly mediates methylation of the cytoplasmic integrin adaptor talin, disrupting the binding of talin to F-actin, thereby promoting the extravasation and motility of DCs under inflammatory conditions. The long-noncoding RNA lnc-Dpf3 directly binds to the transcription factor HIF-1α and suppresses HIF-1α-dependent transcription of the glycolytic gene Ldha, thus inhibiting DC glycolytic metabolism and migratory capacity. (3) Metabolic remodeling (shown in green boxes and arrows). Glycolytic metabolism is essential for CCR7 oligomerization and DC migration, and glycolytic NAD+ supports DC migration by maintaining F-actin polarization and polymerization. Resolvin E1 inhibits DC motility and migration and attenuates the contact hypersensitivity response by disrupting actin polymerization. (4) Cytoskeleton rearrangement (shown in dark red boxes and arrows). Actin polymerization and retrograde flow at the front and actinomyosin contraction at the rear determine cell polarity and provide the driving forces of DC migration. The phosphoinositide transferase protein TIPE2 plays dual roles in signaling regulation by inhibiting Rac-dependent actin polymerization at the rear but enhancing PI3K-dependent actin polymerization at the front, thus facilitating cytoskeleton remodeling and leading-edge formation. The ionic channel TRPML1 (transient receptor potential cation channel, mucolipin subfamily, member 1) is activated for lysosomal calcium release, leading to rearrangement of the actin-based motor protein myosin II at the cell rear, promoting fast and directional migration