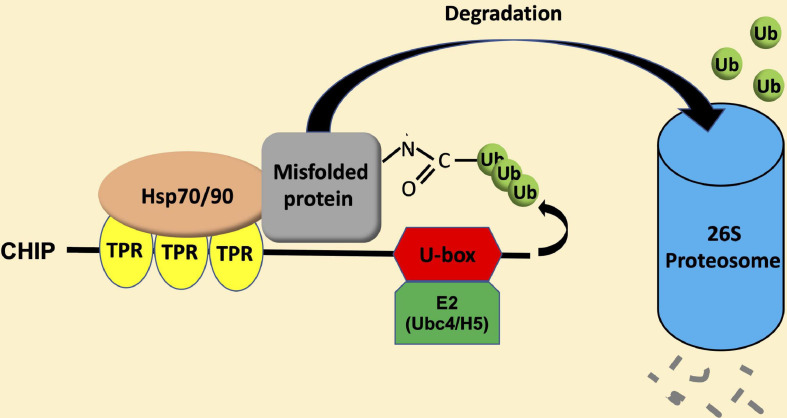
FIGURE 2.
CHIP-mediated ubiquitination and degradation of misfolded proteins. Heat shock protein (Hsp) 70 and Hsp90 recognize misfolded proteins and can switch their role as molecular chaperones from promoting protein folding/maturation to facilitate protein degradation upon binding to the N-terminal TPR domain of CHIP. Upon Hsp70 or Hsp90, CHIP recruits E2 enzymes of the Ubc4/H5 family to ubiquitinate misfolded proteins, and targets their degradation by the 26S proteasome system.