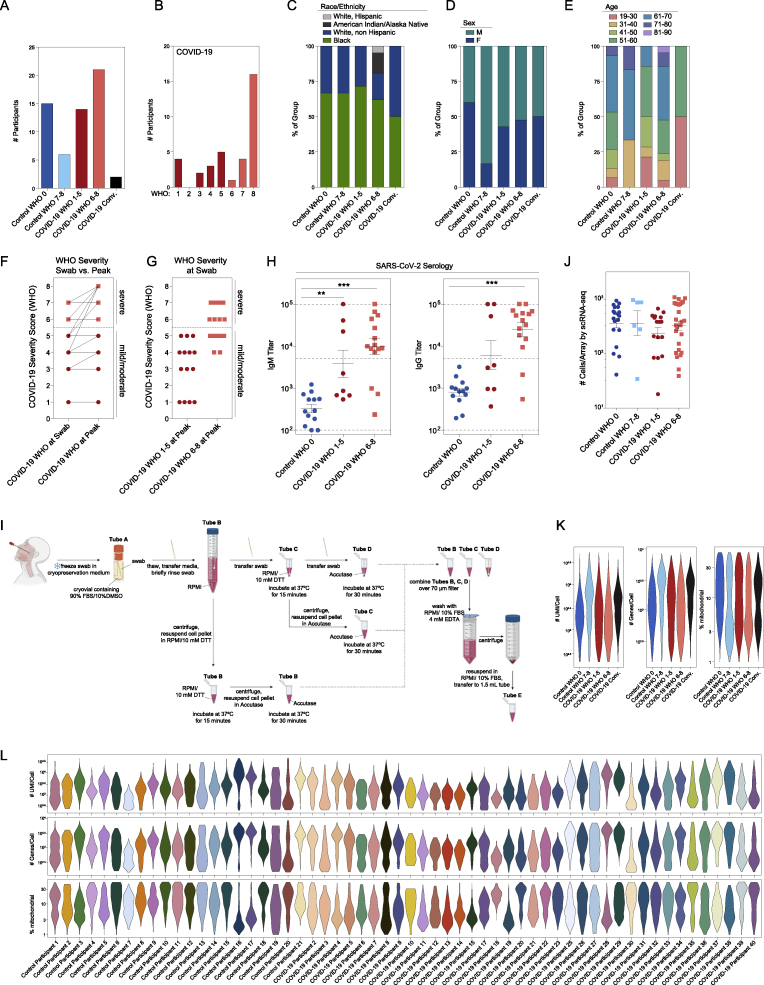
Figure S1.
Cohort and cellular composition of nasopharyngeal swabs, related to Figure 1, Table 1
(A–E) Cohort composition and participant demographics (see also Table 1).
(A) Number of participants by disease group and peak WHO severity score. Dark blue: healthy individuals, Control WHO 0; light blue: Non-COVID-19 ICU/respiratory failure, Control WHO 7-8; red: COVID-19 mild/moderate, COVID-19 WHO 1-5; pink: COVID-19 severe, COVID-19 WHO 6-8; black: recent COVID-19, convalescent.
(B) Number of participants by WHO severity score, COVID-19 participants only.
(C) Participant race and ethnicity by disease group.
(D) Participant sex by disease group.
(E) Participant age by disease group
(F and G) Comparison of WHO severity at swab and peak. WHO severity score among COVID-19 participants at swab (left) and peak (right) (F). WHO severity at swab (G). Red circles: COVID-19 mild/moderate (WHO 1-5) at peak. Pink squares: COVID-19 severe (WHO 6-8) at peak.
(H) SARS-CoV-2 serology: IgM (left) and IgG (right) titers from a subset of Control WHO 0 (blue circles, n = 13) and COVID-19 (red circles, mild/moderate: n = 8; pink squares, severe: n = 15) participants. Plasma samples taken on same day of nasopharyngeal swab. Statistical testing by Kruskal-Wallis test with Dunn’s post hoc testing. Asterisks represent results from Dunn’s test: ∗∗p < 0.01, ∗∗∗p < 0.001. Dashed lines: lower limit of detection: 100; upper limit of detection: 100,000; positive threshold: 5,000.
(I) Detailed schematic of sample preparation and cell processing from nasal swabs (created with BioRender).
(J) Number of high-quality cells/array recovered for single-cell RNA-seq by disease group. Statistical testing by Kruskal-Wallis test (p = 0.37) with Dunn’s post hoc testing, all p > 0.05.
(K) Single-cell quality metrics by group (after filtering for low-quality cells, see STAR Methods).
(L) Single-cell quality metrics by participant (after filtering for low quality cells).