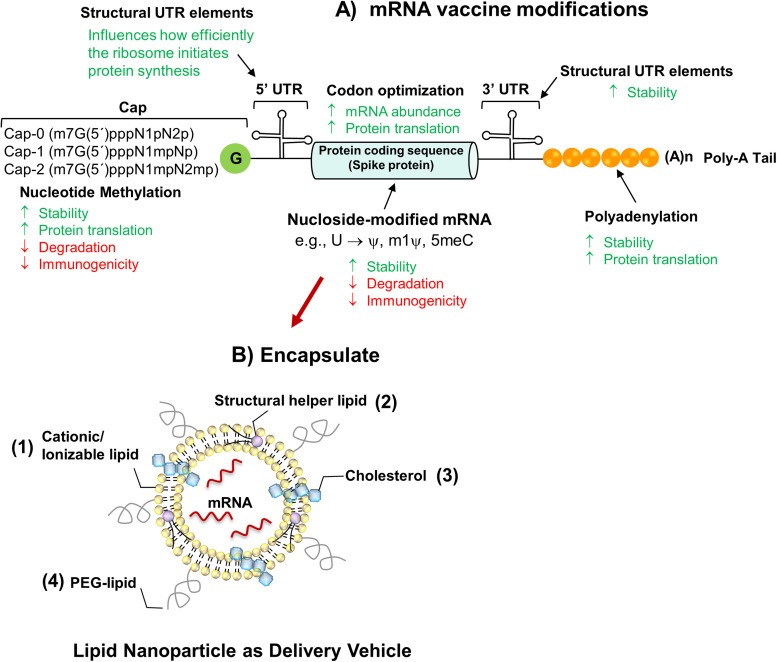
Fig. 1.
Design of the nucleoside-modified SARS-CoV-2 mRNA-LNP vaccines. A) Design of the nucleoside-modified SARS-CoV-2 mRNA-LNP vaccines. A) The critical structures of mRNA are the 5ʹ cap (e.g., the 7–methylguanosine cap), the 5ʹ and 3ʹ untranslated regions (UTRs), sequence encoding the full-length S protein and the poly(A) tail. mRNA cap is incorporated either in one step during transcription in the presence of CAP analogs (e.g., Clean-Cap) or in two steps, after IVT-mRNA production, by enzymatic capping reaction. Replacement of native nucleosides in in-vitro-transcribed mRNA with chemically modified versions reduces immunogenicity and increases translation efficiency. mRNA-1273 and BNT162b2 are nucleoside-modified transcripts with substitution of uridines for N1-methyl pseudouridine is (1mψ). Each of these structural elements of mRNA can be optimized and modified to modulate the stability, translation capacity, and immune-stimulatory profile of mRNA. B) Schematic depiction of mRNA vaccine encapsulated into LNP formulations for improved in vivo mRNA delivery, which are typically composed of (1) an ionizable or cationic lipid [e.g., SM-102 (mRNA-1273) and ALC-0315 (BNT162b2)], bearing tertiary or quaternary amines to encapsulate the polyanionic mRNA; (2) a helper lipid [LNPs of Moderna and BioNTech contain the same helper lipid 1,2-distearoyl-snglycero-3-phosphocholine (DSPC)] that resembles the lipids in the cell membrane; (3) cholesterol to stabilize the lipid bilayer of the LNP; and (4) a polyethylene glycol (PEG)-lipid [(2-[(polyethylene glycol)− 2000]-N,N-ditetradecylacetamide (PEG2000-DMA) in BNT162b2 or 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000 (PEG2000-DMG) in mRNA-1273] to lend the nanoparticle a hydrating layer, improve colloidal stability, and reduce protein absorption.