Abstract
Osteosarcoma is a highly aggressive malignant tumor, which most commonly occurs in children and adolescents. This study aims to reveal that hypoxia promotes the invasion of osteosarcoma cells by up-regulating the expression of NUSAP1. The expression of HIF-1α and NUSAP1 was significantly up-regulated in MG63 cells cultured in hypoxia for 6–36 h. Furthermore, hypoxia induced the migration and invasion of MG63 cells and regulated the level of E-cad, N-cad, Vimentin, Snail, Slug, MMP2, and MMP9 proteins. Importantly, knockdown of NUSAP1 inhibited hypoxia-induced cell migration and invasion. In the hypoxia microenvironment, the addition of HIF-1α inhibitor or the transfection of siRNA specifically targeting HIF-1α significantly reduced the expression of HIF-1α and NUSAP1 and markedly inhibited the migration and invasion of MG63 cells under the hypoxia microenvironment. In conclusion, hypoxia induced the expression of NUSAP1 in a HIF-1α-dependent manner, stimulating the migration and invasion of MG63 cells.
Keywords: osteosarcoma, hypoxia, HIF-1α, NUSAP1, migration, invasion
1. Introduction
Osteosarcoma is a highly aggressive malignant tumor, which most commonly occurs in children and adolescents [1]. The combination of neoadjuvant chemotherapy and traditional surgical resection is the main treatment strategy for osteosarcoma, which improves the overall survival of patients with local osteosarcoma [2]. However, osteosarcoma has a high recurrence rate and is prone to lung metastasis. For metastatic and recurrent osteosarcoma, the combination of surgery and chemotherapy cannot produce satisfactory outcomes [3]. In other words, the clinical outcome of patients with osteosarcoma has not improved significantly. This stagnation of therapeutic advances may be attributed to the unclear mechanism of the osteosarcoma occurrence and metastasis [4]. Therefore, understanding the specific mechanisms of biomolecules in the occurrence and metastasis of osteosarcoma is important to improve the prognosis of patients with osteosarcoma.
Intratumoral hypoxia is a typical feature of solid tumors [5]. This is mainly due to the increase in oxygen consumption caused by the rapid growth of tumor mass and the limited blood supply caused by newly formed vascular malformations. Hypoxia is a vital component of the tumor microenvironment, which is closely related to cell proliferation, tumor invasion, angiogenesis, and distant metastasis [6,7]. The adaptation of tumor cells to hypoxia led to the selection of tumor heterogenous and resistant clones, which evolved into more aggressive phenotypes and resistance to multiple therapeutic drugs [8]. Tumor metastasis and drug resistance caused by hypoxia have also been demonstrated in osteosarcoma [9,10]. However, further understanding of hypoxia-driven metastasis mechanisms is needed.
Nucleolar and spindle-associated protein 1 (NUSAP1) can control the cell cycle progression by promoting the accumulation of microtubules [11,12,13]. The high expression of NUSAP1 has been found in a variety of tumor types and is closely related to tumor cell proliferation, apoptosis, and drug resistance [14,15,16]. However, the role of NUSAP1 in the hypoxia response of osteosarcoma has not been reported. Although, one study has found that hypoxic stress stimulates the rapid translation of NUSAP1 in pancreatic cancer cells [17].
In this study, we found that hypoxia induced the expression of NUSAP1, thereby stimulating the migration and invasion of MG63 cells. Additionally, hypoxia-induced NUSAP1 expression and MG63 cell migration and invasion are HIF-1α dependent.
2. Materials and methods
2.1. Cell lines and cell culture
The human osteosarcoma cell line MG63 was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA), 100 U/mL penicillin, and 100 mg/mL streptomycin. For the normoxic culture, cells were incubated at 37 °C with 5% CO2, 20% O2, and 75% N2 in a humidified incubator (Thermo Fisher Scientific, Waltham, MA). For the hypoxia culture, cells were exposed at 37°C with 1% O2, 5% CO2, and 94% N2 in a humidified incubator.
2.2. Cell transfection and treatment
MG63 cells were transfected with siRNA targeting NUSAP1 or negative control siRNA (Ruibo, Guangzhou, China) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. HIF-1α inhibitor (LW6) was obtained from MedChemExpress (Cat. No. HY-13671).
2.3. Transwell assay
The migration and invasion abilities of MG63 cells were detected by the Transwell assay. For cell migration, 2,000 cells maintained in 200 μL of serum-free medium were seeded in the upper well of the Transwell chamber (8 μm pore size; Corning, Shanghai, China). About 600 μL of DMEM containing 10% FBS was loaded into the lower well. After 24 h of incubation, cells that did not migrate through the pores were carefully wiped with a cotton swab. The cells located on the lower surface of the chamber were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 30 min at room temperature. The stained cells were counted under a light microscope (Olympus, Tokyo, Japan) from five random fields. For cell invasion, the upper chamber was coated with the Matrigel (BD Biosciences, San Jose, CA). Then, the other operations were consistent with cell migration experiments.
2.4. Western blotting
Proteins were lysed from MG63 cells with RIPA buffer containing the protease and phosphatase inhibitors and quantified using the BCA assay (Beyotime, Shanghai, China). An equal amount of protein from each sample was loaded on a 10% SDS-PAGE gel and electrophoresed. Then, the proteins were transferred to a PVDF membrane (EMD Millipore, Billerica, MA), following block with 5% fat-free milk for 1 h at room temperature. The blots were incubated with primary antibodies overnight at 4°C. After washing three times with TBST, the membrane was incubated with HRP-conjugated secondary antibodies, and the immunoblots were visualized using ECL detection kit (Thermo Fisher Scientific). Software QUANTITY ONE was used to measure the intensity of bands. β-actin was used as the reference.
The primary antibodies used in this study were as follow: anti-hypoxia-inducible factor 1α (HIF-1α) (ab1, mouse monoclonal), anti-NUSAP1 (ab169083, mouse polyclonal), anti-N-cadherin (ab18203, rabbit polyclonal), anti-Vimentin (ab8978, mouse monoclonal), anti-Snail (ab229701, rabbit monoclonal), anti-Slug (ab51772, mouse monoclonal), anti-MMP2 (ab92536, rabbit monoclonal), anti-MMP9 (ab38898, rabbit polyclonal), and anti-β-actin (ab8226, mouse monoclonal) were purchased from Abcam. Anti-E-cadherin (20874-1-AP, rabbit polyclonal) was purchased from Proteintech.
2.5. Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA). All data were expressed as means ± SEM from three or more independent experiments. The differences between the groups were determined by Student’s t-test or one-way ANOVA and considered significant at P < 0.05.
3. Results
3.1. Hypoxia induces NUSAP1 expression in MG63 cells
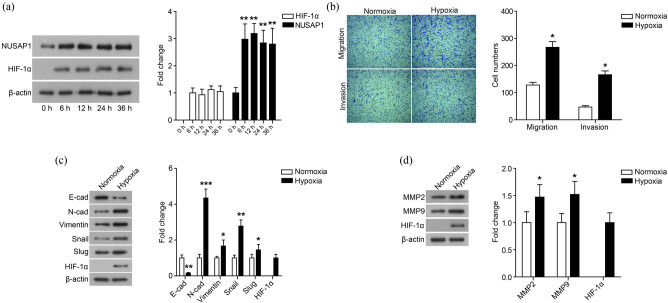
We investigated the effects of hypoxia on the expression of NUSAP1 in human osteosarcoma cell line MG63. As shown in Figure 1a, the expression of NUSAP1 was significantly up-regulated in MG63 cells cultured in hypoxia for 6–36 h. In addition, the level of HIF-1α increased significantly under the hypoxia microenvironment for 6–36 h.
Figure 1.
Hypoxia induces NUSAP1 expression and stimulates the migration and invasion of MG63 cells. (a) The expression of HIF-1α and NUSAP1 in MG63 cells cultured with hypoxia for 0, 6, 12, 24, and 36 h was detected using western blot. (b) The migration and invasion of MG63 cells cultured with normoxia or hypoxia for 24 h was measured using transwell assay. (c) The expression of EMT-related proteins in MG63 cells cultured with normoxia or hypoxia for 48 h was detected using western blot. (d) The expression of MMP family membranes in MG63 cells cultured with normoxia or hypoxia for 48 h was detected using western blot. All experiments were independently carried out in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001, compared with normoxia group.
3.2. Hypoxia stimulates MG63 cell migration and invasion
Subsequently, the effect of hypoxia on the migration and invasion of MG63 cells was measured by the Transwell assay. As shown in Figure 1b, the number of migrating and invading cells increased after exposure to hypoxia (P < 0.05). Furthermore, the hypoxia microenvironment regulated the expression of EMT-related proteins. As shown in Figure 1c, the hypoxia microenvironment down-regulated the expression of E-cad of MG63 cells, while up-regulating the level of N-cad, Vimentin, Snail, and Slug (P < 0.05). We also found that the expression of MMP2 and MMP9 significantly increased under the hypoxia microenvironment (Figure 1d, P < 0.05).
3.3. Knockdown of NUSAP1 represses the migration and invasion of MG63 cells
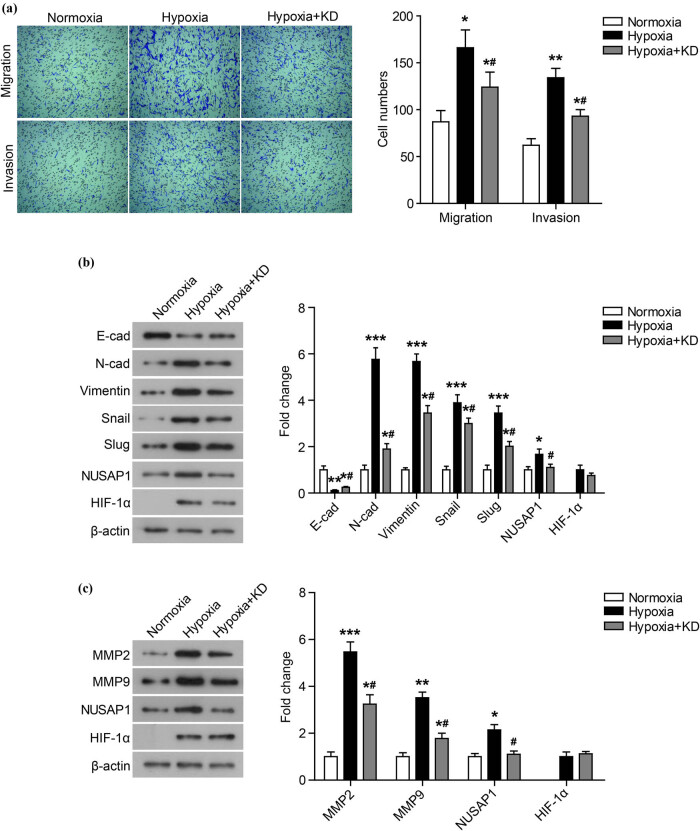
To investigate whether hypoxia-induced cell migration and invasion are related to the hypoxia-induced high expression of NUSAP1, NUSAP1 expression was knocked down by transfection of siRNA-NUSAP1 under hypoxia condition. As shown in Figure 2a, after knocking down the NUSAP1 expression, the number of migrating and invading cells under hypoxia conditions was markedly reduced. Knockdown of NUSAP1 significantly suppressed the expression of N-cad, Vimentin, Snail, Slug, MMP2, and MMP9, while it markedly promoted the expression of E-cad (Figure 2b and c, P < 0.05).
Figure 2.
Knockdown of NUSAP1 represses the migration and invasion of MG63 cells under hypoxia. (a) The migration and invasion of MG63 cells whose NUSAP1 expression was knocked down (Hypoxia + KD) under hypoxia condition was measured using transwell assay. (b) The expression of EMT-related proteins was detected using western blot. (c) The expression of MMP family membranes was detected using western blot. All experiments were independently carried out in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001, compared with normoxia group; # P < 0.05, compared with Hypoxia group.
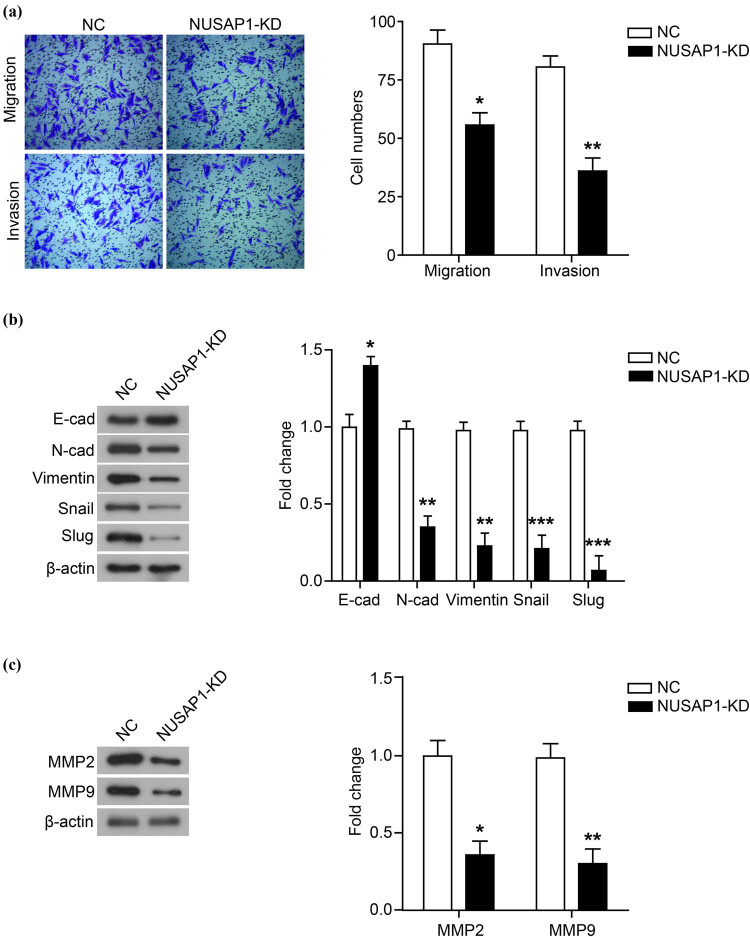
In addition, we also measured the effect of down-regulation of NUSAP1 expression on MG63 cell migration and invasion under normoxia. As shown in Figure 3, knockdown of NUSAP1 inhibited cell migration and invasion and regulated the expression of EMT-related proteins and MMP proteins.
Figure 3.
Knockdown of NUSAP1 represses the migration and invasion of MG63 cells. (a) The migration and invasion of MG63 cells whose NUSAP1 expression was knocked down (NUSAP1-KD) under normoxia condition was measured using transwell assay. (b) The expression of EMT-related proteins was detected using western blot. (c) The expression of MMP family membranes was detected using western blot. All experiments were independently carried out in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001, compared with NC group.
3.4. Hypoxia increases NUSAP1 expression and MG63 cell migration and invasion is HIF-1α dependent
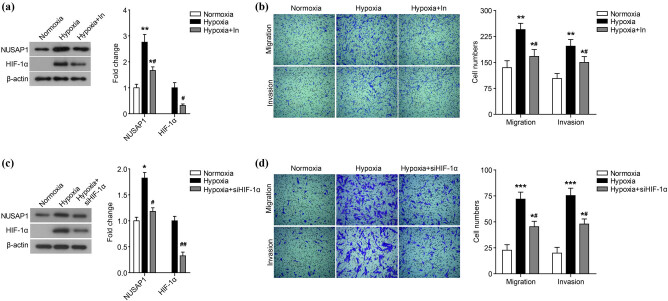
HIF-1α is the central transcription factor that regulates the transcription of hypoxia-responsive genes and the adaptive response of cells to hypoxia. Therefore, we further evaluated whether the hypoxia-induced up-regulation of NUASP1 expression is HIF-1α dependent. In the hypoxia microenvironment, the addition of HIF-1α inhibitor (Figure 4a) and the transfection of siRNA specifically targeting HIF-1α (siHIF-1α) (Figure 4c) can significantly reduce the expression of HIF-1α and NUSAP1 (P < 0.05). Additionally, the addition of HIF-1α inhibitor and the transfection of siHIF-1α both markedly inhibited the cell migration and invasion under the hypoxia microenvironment (Figure 4b and d, P < 0.05).
Figure 4.
Hypoxia increases NUSAP1 expression and MG63 cell migration and invasion is HIF-1α dependent. The expression of HIF-1α and NUSAP1 in MG63 cells incubated with HIF-1α inhibitor (Hypoxia + In) (a) or siRNA targeting HIF-1α (siHIF-1α) (c) under hypoxia condition was detected using western blot. The migration and invasion of MG63 cells incubated with HIF-1α inhibitor (b) or siRNA targeting HIF-1α (siHIF-1α) (d) under hypoxia condition was measured using transwell assay. All experiments were independently carried out in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001, compared with normoxia group; # P < 0.05, compared with Hypoxia group.
4. Discussion
Osteosarcoma is a common primary malignant tumor, accounting for more than 10% of solid cancers in children and adolescents [18]. Although the treatment of osteosarcoma has made great progress in the past 20 years, the overall survival rate of osteosarcoma patients has not improved due to metastasis and recurrence [19]. Therefore, it is necessary to understand its underlying biological reasons to improve the outcome of osteosarcoma treatment. In this study, we found that hypoxia can promote the migration and invasion of osteosarcoma cells by up-regulating the expression of NUSAP1. These findings may provide a valuable target for the treatment of osteosarcoma.
Hypoxia is an important prognostic and driven factor for many types of tumors [20,21]. In this study, we found that hypoxia induced the expression of NUSAP1. Furthermore, one study has shown that hypoxia stimulates the rapid translation of NUSAP1 in pancreatic cancer cells [17]. Additionally, we also found that hypoxia promoted the migration and invasion of osteosarcoma cells and regulated the expression of EMT- and MMP-related proteins. Hypoxia-induced cell migration and invasion were related to the high expression of NUSAP1 induced by hypoxia. After knocking down the expression of NUSAP1, the number of migrating and invading cells under hypoxia was markedly reduced.
The members of the HIF family are oxygen sensors that mediate the response of mammalian cells to hypoxia [22]. The members of this family contain an oxygen-sensitive HIF-α subunit and a constitutively expressed HIF-β subunit [22]. HIF-1α is widely used as a marker of poor prognosis in cancer patients. It can act as a signaling center, transcriptionally regulating the expression of many transcription factors and signaling molecules that play a key role in tumorigenesis [22]. Additionally, HIF-1α plays an important role in the response of cells to hypoxia by inducing glycolysis and angiogenesis [23]. Some studies have also shown that HIF-1a is up-regulated in osteosarcoma and is associated with the metastasis and poor prognosis [5,24,25]. In this study, we found that the increased NUSAP1 expression and MG63 cell migration and invasion induced by hypoxia were HIF-1α dependent. The results of the western blot showed that the addition of HIF-1α inhibitor or the transfection of siRNA targeting HIF-1α significantly reduced the expression of HIF-1α and NUSAP1 in the hypoxia microenvironment and markedly inhibited the cell migration and invasion. We speculated that NUSAP1 was the downstream target of HIF-1α transcriptional regulation and participates in the regulation of osteosarcoma cell migration and invasion in a HIF-1α-dependent manner.
We reported for the first time the role of NUSAP1 in osteosarcoma, which is consistent with its role in other types of tumors. NUSAP1 plays an oncogene role in tumors, which is mainly involved in regulating tumor cell proliferation and apoptosis [14,15,16]. This study also demonstrates the link between NUSAP1 and tumor cell migration and invasion.
In conclusion, hypoxia induced the NUSAP1 expression in a HIF-1α-dependent manner in MG63 cells, which stimulated the migration and invasion. HIF-1α and NUSAP1 were valuable targets for the treatment of osteosarcoma.
Acknowledgments
The authors declare that they have no competing interests, and all authors should confirm its accuracy.
Footnotes
Funding information: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13(4):357–68. [DOI] [PubMed]; Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13(4):357–68. doi: 10.2217/fon-2016-0261. [DOI] [PubMed] [Google Scholar]
- [2].Simpson S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59(1):71. [DOI] [PMC free article] [PubMed]; Simpson S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59(1):71. doi: 10.1186/s13028-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–92. [DOI] [PubMed]; Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–92. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- [4].Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog. 2015;20(3–4):173–97. [DOI] [PMC free article] [PubMed]; Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog. 2015;20(3–4):173–97. doi: 10.1615/critrevoncog.2015013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang B, Li YL, Zhao JL, Zhen O, Yu C, Yang BH, et al. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma. Biomed Pharmacother. 2018;105:1–9. [DOI] [PubMed]; Zhang B, Li YL, Zhao JL, Zhen O, Yu C, Yang BH. et al. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma. Biomed Pharmacother. 2018;105:1–9. doi: 10.1016/j.biopha.2018.03.165. [DOI] [PubMed] [Google Scholar]
- [6].Sun Y, Wang H, Liu M, Lin F, Hua J. Resveratrol abrogates the effects of hypoxia on cell proliferation, invasion and EMT in osteosarcoma cells through downregulation of the HIF-1alpha protein. Mol Med Rep. 2015;11(3):1975–81. [DOI] [PubMed]; Sun Y, Wang H, Liu M, Lin F, Hua J. Resveratrol abrogates the effects of hypoxia on cell proliferation, invasion and EMT in osteosarcoma cells through downregulation of the HIF-1alpha protein. Mol Med Rep. 2015;11(3):1975–81. doi: 10.3892/mmr.2014.2913. [DOI] [PubMed] [Google Scholar]
- [7].Chang J, Erler J. Hypoxia-mediated metastasis. Adv Exp Med Biol. 2014;772:55–81. [DOI] [PubMed]; Chang J, Erler J. Hypoxia-mediated metastasis. Adv Exp Med Biol. 2014;772:55–81. doi: 10.1007/978-1-4614-5915-6_3. [DOI] [PubMed] [Google Scholar]
- [8].Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32. [DOI] [PubMed]; Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- [9].Zhao C, Zhang Q, Yu T, Sun S, Wang W, Liu G. Hypoxia promotes drug resistance in osteosarcoma cells via activating AMP-activated protein kinase (AMPK) signaling. J Bone Oncol. 2016;5(1):22–9. [DOI] [PMC free article] [PubMed]; Zhao C, Zhang Q, Yu T, Sun S, Wang W, Liu G. Hypoxia promotes drug resistance in osteosarcoma cells via activating AMP-activated protein kinase (AMPK) signaling. J Bone Oncol. 2016;5(1):22–9. doi: 10.1016/j.jbo.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao J, Wang Y, Dong R, Lin G, Zhang N, Wang J, et al. Hypoxia-induced WSB1 promotes the metastatic potential of osteosarcoma cells. Cancer Res. 2015;75(22):4839–51. [DOI] [PubMed]; Cao J, Wang Y, Dong R, Lin G, Zhang N, Wang J. et al. Hypoxia-induced WSB1 promotes the metastatic potential of osteosarcoma cells. Cancer Res. 2015;75(22):4839–51. doi: 10.1158/0008-5472.CAN-15-0711. [DOI] [PubMed] [Google Scholar]
- [11].Li C, Zhang Y, Yang Q, Ye F, Sun SY, Chen ES, et al. NuSAP modulates the dynamics of kinetochore microtubules by attenuating MCAK depolymerisation activity. Sci Rep. 2016;6:18773. [DOI] [PMC free article] [PubMed]; Li C, Zhang Y, Yang Q, Ye F, Sun SY, Chen ES. et al. NuSAP modulates the dynamics of kinetochore microtubules by attenuating MCAK depolymerisation activity. Sci Rep. 2016;6:18773. doi: 10.1038/srep18773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li C, Xue C, Yang Q, Low BC, Liou YC. NuSAP governs chromosome oscillation by facilitating the Kid-generated polar ejection force. Nat Commun. 2016;7:10597. [DOI] [PMC free article] [PubMed]; Li C, Xue C, Yang Q, Low BC, Liou YC. NuSAP governs chromosome oscillation by facilitating the Kid-generated polar ejection force. Nat Commun. 2016;7:10597. doi: 10.1038/ncomms10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vanden Bosch A, Raemaekers T, Denayer S, Torrekens S, Smets N, Moermans K, et al. NuSAP is essential for chromatin-induced spindle formation during early embryogenesis. J Cell Sci. 2010;123(Pt 19):3244–55. [DOI] [PubMed]; Vanden Bosch A, Raemaekers T, Denayer S, Torrekens S, Smets N, Moermans K. et al. NuSAP is essential for chromatin-induced spindle formation during early embryogenesis. J Cell Sci. 2010;123(Pt 19):3244–55. doi: 10.1242/jcs.063875. [DOI] [PubMed] [Google Scholar]
- [14].Zhang X, Pan Y, Fu H, Zhang J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression. Med Sci Monit. 2018;24:8553–64. [DOI] [PMC free article] [PubMed]; Zhang X, Pan Y, Fu H, Zhang J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5 expression. Med Sci Monit. 2018;24:8553–64. doi: 10.12659/MSM.910364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gordon CA, Gong X, Ganesh D, Brooks JD. NUSAP1 promotes invasion and metastasis of prostate cancer. Oncotarget. 2017;8(18):29935–50. [DOI] [PMC free article] [PubMed]; Gordon CA, Gong X, Ganesh D, Brooks JD. NUSAP1 promotes invasion and metastasis of prostate cancer. Oncotarget. 2017;8(18):29935–50. doi: 10.18632/oncotarget.15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu T, Xie P, Gao YF, Huang MS, Li X, Zhang W, et al. Nucleolar and spindle-associated protein 1 is a tumor grade correlated prognosis marker for glioma patients. CNS Neurosci Ther. 2018;24(3):178–86. [DOI] [PMC free article] [PubMed]; Zhu T, Xie P, Gao YF, Huang MS, Li X, Zhang W. et al. Nucleolar and spindle-associated protein 1 is a tumor grade correlated prognosis marker for glioma patients. CNS Neurosci Ther. 2018;24(3):178–86. doi: 10.1111/cns.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gupta N, Park JE, Tse W, Low JK, Kon OL, McCarthy N, et al. ERO1alpha promotes hypoxic tumor progression and is associated with poor prognosis in pancreatic cancer. Oncotarget. 2019;10(57):5970–82. [DOI] [PMC free article] [PubMed]; Gupta N, Park JE, Tse W, Low JK, Kon OL, McCarthy N. et al. ERO1alpha promotes hypoxic tumor progression and is associated with poor prognosis in pancreatic cancer. Oncotarget. 2019;10(57):5970–82. doi: 10.18632/oncotarget.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bielack S, Carrle D, Casali PG, Group EGW. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):137–9. [DOI] [PubMed]; Bielack S, Carrle D, Casali PG, Group EGW. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):137–9. doi: 10.1093/annonc/mdp154. [DOI] [PubMed] [Google Scholar]
- [19].Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–56. [DOI] [PubMed]; Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–56. doi: 10.1586/14737140.2016.1168697. [DOI] [PubMed] [Google Scholar]
- [20].Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. [DOI] [PMC free article] [PubMed]; Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175–80. [DOI] [PMC free article] [PubMed]; Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175–80. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Manoochehri Khoshinani H, Afshar S, Najafi R. Hypoxia: a double-edged sword in cancer therapy. Cancer Invest. 2016;34(10):536–45. [DOI] [PubMed]; Manoochehri Khoshinani H, Afshar S, Najafi R. Hypoxia: a double-edged sword in cancer therapy. Cancer Invest. 2016;34(10):536–45. doi: 10.1080/07357907.2016.1245317. [DOI] [PubMed] [Google Scholar]
- [23].Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94(15):8104–9. [DOI] [PMC free article] [PubMed]; Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ. et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94(15):8104–9. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ren HY, Zhang YH, Li HY, Xie T, Sun LL, Zhu T, et al. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: a meta-analysis. Onco Targets Ther. 2016;9:1477–87. [DOI] [PMC free article] [PubMed]; Ren HY, Zhang YH, Li HY, Xie T, Sun LL, Zhu T. et al. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: a meta-analysis. Onco Targets Ther. 2016;9:1477–87. doi: 10.2147/OTT.S95490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao H, Wu Y, Chen Y, Liu H. Clinical significance of hypoxia-inducible factor 1 and VEGF-A in osteosarcoma. Int J Clin Oncol. 2015;20(6):1233–43. [DOI] [PubMed]; Zhao H, Wu Y, Chen Y, Liu H. Clinical significance of hypoxia-inducible factor 1 and VEGF-A in osteosarcoma. Int J Clin Oncol. 2015;20(6):1233–43. doi: 10.1007/s10147-015-0848-x. [DOI] [PubMed] [Google Scholar]