Summary
Autoimmune hepatitis (AIH) is a relatively rare non-resolving chronic liver disease, which mainly affects women. It is characterized by hypergammaglobulinemia, circulating autoantibodies, interface hepatitis on liver histology and a favourable response to immunosuppression. The putative mechanism for the development of autoimmune hepatitis is thought to be the interaction between genetic predisposition, environmental triggers and failure of the native immune system.
AIH still remains a major diagnostic and therapeutic challenge, mainly because it is a very heterogeneous disease. Prompt and timely diagnosis is crucial since, if left untreated, AIH has a high mortality rate. Histological demonstration of hepatitis is required for the diagnosis of AIH and, therefore, liver biopsy is mandatory in the initial diagnostic work-up, before treatment. In this review, we summarize the histological features of AIH with the main aim of highlighting the most important clinical-pathological hallmarks useful in the routine diagnostic practice.
Key words: autoimmune hepatitis, histology, liver biopsy, interface hepatitis
Introduction
Autoimmune hepatitis (AIH) is a chronic progressive liver disease of unknown etiology. The pathogenesis of AIH is complex and involves interactions between tolerant liver, environmental triggers, and dysregulated immunological mechanisms. Genetic factors influence an individual’s susceptibility to developing AIH 1,2.
Originally considered a disease of young women, AIH may also occur in children and the elderly (about 30% of cases occur over 60 years of age). Both sexes (about 30% are males) and all ethnic groups are involved, and recent epidemiological studies indicate an increasing trend in AIH prevalence worldwide, especially in men 3. If untreated, AIH leads to cirrhosis and associated complications, with a small (1-2% per year)but significant risk of hepatocellular carcinoma development 4,5. Characteristic laboratory features include hypergammaglobulinemia, with elevation of immunoglobulin G (IgG) in the majority of cases, and presence of non-organ specific autoantibodies 6.
Diagnosis is often challenging, since no single feature is reliable. Therefore, careful clinical-pathological integration is required for the exclusion of other causes of liver disease and a confident diagnosis of AIH. An early diagnosis is, however, critical for timely initiation of life-saving immunosuppressive therapy.
Current guidelines recommend liver biopsy as a prerequisite for the diagnosis of AIH 1. Liver biopsy is also useful for treatment management, to determine disease severity and distinguish acute vs. chronic presentation.
This review focuses on the current role of liver biopsy and describes characteristic histological lesions which contribute to AIH diagnosis.
Overview on laboratory and clinical features
Liver function tests typically show a hepatocellular pattern of injury, with an increase in aminotransferases, that can be mildly elevated or up to 50 times the upper normal value. Alanine aminotransferase (ALT) is typically higher than Aspartate aminotransferase (AST). Cholestatic enzymes are usually normal or mildly elevated unless there is an overlap with primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC) 7-13. Increase in serum globulins (serum γ-globulin or IgG level) is evident in 90% of patients. This prevalence seems to be lower in patients with an acute onset, among which a proportion of cases, ranging from 25 to 39%, has normal IgG levels 14,15. IgA and IgM are usually normal, and their increased levels should prompt the exclusion of different etiologies, such as alcoholic steatohepatitis and PBC, respectively1.
Circulating autoantibodies are considered the hallmark of AIH and have been used to subclassify AIH into type 1, or classic autoimmune hepatitis, and type 2. Type 1 AIH is defined by the presence of antinuclear antibody (ANA) and/or anti-smooth muscle antibody (ASMA). Antibody titers more than 1:40 are considered significant in adults. Type 2 AIH is less common and mainly affects pediatric patients. It is characterized by the presence of antibodies against liver/kidney microsomes (LKM1) and/or liver cytosol antigen (LC1). The validity of such subclassification in clinical practice has been questioned 1. Autoantibodies may not be detected, particularly in cases of severe acute presentation (autoantibody negative AIH)16. Noteworthy, is that the presence of autoantibodies always needs careful interpretation, as they can be found in healthy patients as well as in other, non-autoimmune, hepatopathies. In asymptomatic blood donors, ANA prevalence of any titer has been found to vary between 4% and 26%, with nearly 15% of cases positive at a 1:40 dilution, and up to 10% of pregnant women are ANA positive 17-19. ANA are found in approximately 5% of individuals with HCV, showing titers more than 1:100 20. The presence of autoantibodies is common also in patients with Non-alcoholic fatty liver disease (NAFLD): in a multicenter study in United States, significant titers of autoantibodies were detectable in 21% of adult NAFLD patients, with no association with advanced histological features or presence of steatohepatitis 21.
The spectrum of clinical presentation of AIH ranges from asymptomatic elevation of serum liver enzymes, to fulminant hepatitis. The most common clinical presentation is characterized by a slow disease onset and progression with non-specific complaints such as fatigue and malaise, which explains why AIH is in advanced stages of fibrosis at the time of diagnosis in around a third of patients. Acute onset of AIH occurs in about one third of patients. It may represent an acute exacerbation of unrecognized AIH with pathological evidence of chronic hepatitis or a genuine newly developed acute AIH, without previous clinical-pathological findings of chronic liver disease. Acute onset is clinically indistinguishable from acute hepatitis of other causes 22-24. Moreover, in some of these patients, IgG levels may be within the normal range and ANA and/or SMA may be negative at the first screening 10,25-28, thus leading to a challenging diagnosis.
The role of liver biopsy in AIH
Liver biopsy is recommended by the American Association for the Study of Liver diseases (AASLD) and the European Association for the Study of the Liver (EASL) guidelines to help establish the diagnosis, exclude other causes of liver disease, and guide treatment choice 1,26.
The diagnostic criteria for AIH have been codified in 1993, revised in 1999 by the International Autoimmune Hepatitis Group (IAHG) 7,8 and more recently simplified for clinical use (Table I) 9. In the simplified system, as in the previous ones, liver histology is included among the parameters required to confirm clinical diagnosis of AIH. Indeed, the system comprises four parameters: autoantibodies, serum IgG, results of viral hepatitis work-up and AIH histology, which is coded as absent, typical or compatible (Table II).
Table I.
Simplified diagnostic criteria for autoimmune hepatitis (from Hennes et al., 2009, adapt.) 9.
| Feature | Cutoff | Points |
|---|---|---|
| ANA or SMA | ≥ 1:40 | 1 |
| ANA or SMA | ≥ 1:80 | 2* |
| or LKM | ≥ 1:40 | |
| or SLA | Positive | |
| IgG | > Upper normal limit | 1 |
| > 1.10 times upper normal limit | 2 | |
| Liver histology (evidence of hepatitis is a necessary condition) | Compatible with AIH Typical AIH |
1 2 |
| Absence of viral hepatitis | Yes | 2 |
Interpretation of aggregated scores: ≥ 6: probable AIH; ≥ 7: definite AIH
*Addition of points achieved for all autoantibodies (maximum, 2 points).
ANA, Antinuclear antibody; SMA, smooth muscle antibody; LKM, liver-kidney microsomal antibody; SLA/LP, soluble liver antigen/liver pancreas antibody, AIH, autoimmune hepatitis.
Table II.
Histological categories for grading histology in the simplified system for autoimmune hepatitis (from Hennes et al., 2009, adapt.) 9.
| Histological Categories | Morphological features |
| Typical for AIH | Interface hepatitis, with lymphocytic/lymphoplasmocytic infiltrate in portal tracts extending into the lobule Emperipolesis Hepatic rosette formation (All three features have to be present) |
| Compatible with AIH | Chronic hepatitis pattern of injury with lymphocytic infiltration Lack of all the features considered typical |
| Atypical for AIH | Signs suggestive of other diagnosis |
AIH, Autoimmune hepatitis.
Once a diagnosis of AIH is made, liver biopsy is the gold standard for grading and staging AIH and provides crucial information for patient management and treatment decisions29. Persistence of any degree of inflammation, particularly interface hepatitis, and presence of plasma cells in biopsy samples taken under treatment, are strong predictors of AIH- relapse if immunosuppression is stopped.
Liver biopsy is particularly important for the differential diagnosis of AIH, as it may lead to an alternative etiology 30. Moreover, it may identify possible concurrent disorders, especially fatty liver disease, considering the epidemic proportion of risk factors for the metabolic syndrome. Finally, liver biopsy is considered mandatory in cases of AIH with overlapping features of autoimmune biliary disorders. Liver biopsy at presentation should be performed prior to beginning treatment since immunosuppression may rapidly clear inflammation on liver biopsy, with the risk of a “false negative” diagnosis.
Histopathology of AIH
Despite the pivotal role of liver biopsy for diagnosis, no morphological feature is pathognomonic of AIH. However, there is a characteristic picture in many patients before treatment. The typical aspect of AIH is that of a severe chronic hepatitis with intense portal and lobular inflammation, severe interface hepatitis, and much hepatocyte damage.
Portal inflammation is composed of mononuclear cells, mainly lymphocytes, with a variable amount of plasma cells. Some eosinophils and neutrophils may be seen (Fig. 1). Plasma cells are considered typical for AIH, but they are neither sufficient nor necessary to make a diagnosis since they are rare/absent in about one third of cases. However, detection of plasma cells in clusters (Fig. 2) is highly suggestive of AIH. In a recent critical appraisal concerning the histological features of AIH 31, the presence of plasma cell clusters (defined as a collection of ≥ 5 plasma cells) in the lobule was the most sensitive diagnostic finding. Immunohistochemical stains for multiple myeloma-1 (MUM-1) or CD38 may help to highlight number and distribution pattern of plasma cells (Fig. 3) 32.
Figure 1.
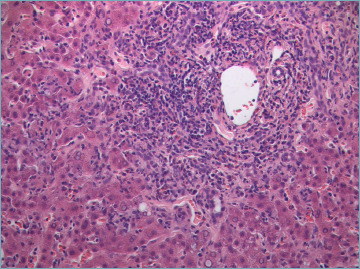
Severe portal inflammation, mainly composed of lymphocytes, and interface hepatitis. Several necro-inflammatory foci are visible in the adjacent lobular parenchyma (H&E; original magnification 40x).
Figure 2.
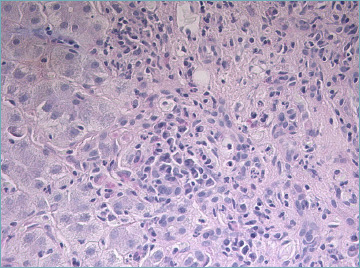
A cluster of plasma cells is visible close to the portal tract (H&E; original magnification 40x).
Figure 3.
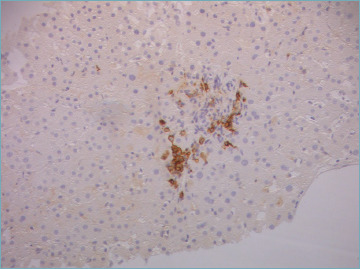
Immunostain with CD38 helps in identifying a cluster of plasma cells. This is a pediatric case with mild portal inflammation within an otherwise typical clinical presentation (original magnification 20x).
Although not specific, interface hepatitis is considered the hallmark of AIH. It is characterized by the extension of portal inflammation beyond the limiting plate into the adjacent lobule with damage and progressive loss of hepatocytes at the portal-lobular interface (Fig. 1). It is observed in up to 98% of AIH and is usually more prominent compared to interface hepatitis of other causes 33. According to the 2008 simplified criteria, the presence of interface hepatitis, even in the absence of all the other typical features, is in agreement with the diagnosis of AIH 9.
Severe portal and interface hepatitis are usually associated with ductular reaction (DR). It consists of bile ductules with poorly defined lumina at the portal-parenchymal interface, arranged in anastomosing cords, and lined by small CK7-positive cells (Fig. 4). Ductular reaction is a regenerative phenomenon, which represents proliferation and bidirectional differentiation of facultative hepatic stem cells in a variety of acute and chronic liver diseases. In AIH, DR correlates with the severity of portal-periportal inflammation, as in other liver diseases 34.
Figure 4.
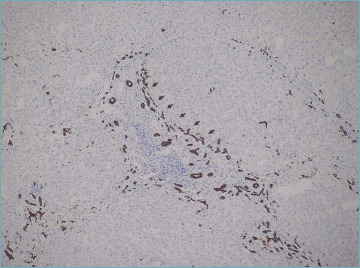
Ductular reaction (arrows) around an inflamed portal tract (immunostain for CK7; original magnification 20x).
Lobular changes are dominated by necro-inflammatory damage, ranging from spotty to confluent necrosis. Apoptotic bodies are commonly seen. Multiple necro-inflammatory foci may be associated with hepatocyte ballooning and sinusoidal inflammation, giving the appearance of lobular disarray (Fig. 5) which resembles what is seen in patients with acute viral hepatitis. Bridging necrosis (portal to portal and portal to central) is not uncommon and may represent the deep extension of interface hepatitis into the lobules. Confluent necrosis and inflammation may be seen in perivenular areas. In most cases, it is associated with the typical portal peri-portal inflammation. However, there are few patients affected by AIH in whom the major feature at presentation is the isolated centrilobular necro-inflammation, with spared portal tract (Fig. 6). This is thought to be an early feature of AIH that precedes overt portal-dominant (classic) AIH 35. Indeed, this pattern of necrosis is commonly seen in patients with acute disease onset 36. In rare cases, a massive-panlobular necrosis may occur, leading to liver failure.
Figure 5.
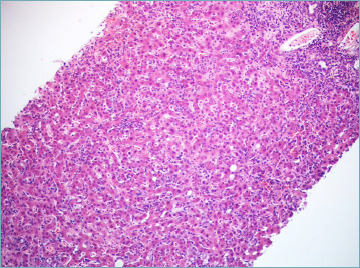
Several foci of “spotty” necrosis, giving the appearance of lobular disarray (H&E; original magnification 20x).
Figure 6.
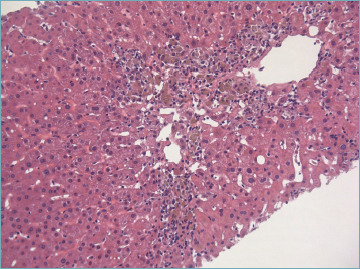
Centrilobular necro-inflammation in a case of true acute AIH presentation. Portal tracts were completely spared in this case. Toxic damage is the main differential diagnosis (H&E; original magnification 40x).
Much emphasis has been given to the presence of rosettes (Fig. 7) and emperipolesis (Fig. 8) as classic changes of AIH. Indeed, they have been considered as typical histological AIH-features in the 2008 simplified scoring system for adults and they are a required item to get score 2 (Tab. II)9. Rosettes are defined as hepatocytes arranged around a central lumen and represent a regenerative response to the necro-inflammatory damage. Emperipolesis is characterized by the presence of a mononucleated inflammatory cell (lymphocyte or plasma cell) within the cytoplasm of hepatocytes and it is reported in 65-78% of AIH cases. Emperipolesis and rosette formation have been considered better histological predictors of AIH, when compared to plasma cells and interface hepatitis 37. However, such superiority is still controversial and it has been shown that emperipolesis is associated with the severity of activity rather than the etiology 38. Moreover, emperipolesis is difficult to be reliably assessed in routine practice with light microscopy, and it is best evaluated by electron microscopy.
Figure 7.
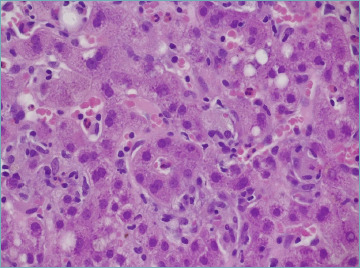
Typical hepatocyte rosette, representing a regenerative phenomenon in a heavily inflamed liver (H&E; original magnification 60x).
Figure 8.
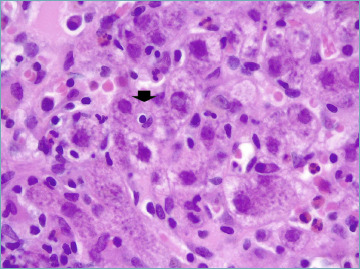
Emperipolesis (arrow) appears as a lymphocyte within the hepatocyte cytoplasm (H&E; original magnification 60x).
Bile duct injury can be identified in up to 83% of AIH patients 34,39,40, often in a PBC-like pattern, even after the exclusion of an overlap syndrome. Therefore, some degree of biliary involvement in AIH does not necessarily lead to a change in diagnosis! A major problem is that how much bile duct damage is diagnostic of biliary disease is still not established. In routine practice, it is reasonable to suggest a possible concomitant biliary disease when bile duct damage is seen in the majority of portal tracts. The bile ducts are not the target of damage in AIH, and probably their inflammatory damage represents collateral injury of the conspicuous inflammatory process, as demonstrated by their subsiding after immunosuppressive therapy 34. Therefore, an evident bile duct destruction (ductopenia) is not a feature of AIH and warrants consideration of primary biliary cholangitis. Moreover, copper-associated protein accumulation and/or CK7-positive periportal hepatocytes are recognized features of chronic cholestasis and their presence should prompt to consider an alternative diagnosis or an overlap condition. It is noteworthy to highlight that these features of chronic cholestasis no longer have their diagnostic value when severe fibrosis or cirrhosis is present. In such cases, nonspecific copper-associated protein accumulation and CK7 positivity can be seen, notwithstanding the etiology. The practical value of taking in consideration results of copper-associated protein and CK7 stains in the histological features has been evaluated in a recent study 41. In this study, histological criteria codified in the 2008 simplified score proposed by the IAHG (Tab. II) were reviewed and new parameters were proposed. The new criteria (Tab. III) were based on interface/lobular inflammatory activity, number of plasma cells, biliary features, and copper-associated protein/CK7 stains, and demonstrated higher sensitivity for the diagnosis of AIH. These results should be validated in prospective series.
Table III.
Proposed criteria for the histologic scoring of autoimmune hepatitis (from Balitzer et al., 2017, adapt.) 41.
| Histologic score | Morphological features |
|---|---|
| Score 0 | Features not observed in AIH (e.g. florid duct lesion, bile duct loss, or copper/CK7 positivity, if applicable*) |
| Score 1** |
|
| Score 2 | Hepatitic pattern with any of the following:
|
*Applicable only in cases without any bridging fibrosis.
**Both 1 and 2 are necessary for histologic score 1, except in cases with acute presentation.
AIH, Autoimmune hepatitis.
Cholestasis is usually not observed in AIH, but a mild degree may be seen in cases with marked lobular inflammation.
Giant cell transformation may be rarely seen in adults with AIH (Fig. 9). This represents an unusual regenerative or degenerative hepatocyte reaction to various injuries, and it is a common response in the newborn liver diseases. Post infantile giant cell hepatitis is a rare, non-specific subtype of hepatitis, which can be seen in a wide variety of inflammatory and cholestatic liver diseases, among which AIH represents the most common cause 42.
Figure 9.
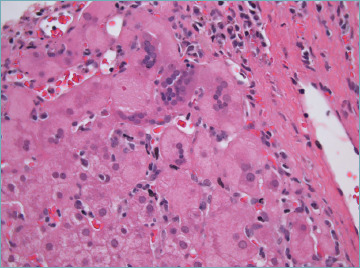
Giant cell transformation in autoimmune hepatitis (H&E; original magnification 40x).
Recently, PAS positive-diastase resistant hyaline droplets in Kupffer cells, resembling Russell bodies of plasma cells, have been described as specific feature in AIH 43. However, little information is known on this and, therefore, it is still controversial to consider this feature as a reliable histological sign of AIH.
Histological grading and staging
Since no specific grading and staging systems for AIH have been developed, grading of the inflammatory activity and staging of the fibrosis can be performed borrowing the systems used for chronic viral hepatitis such as the Scheuer 44, Ishak 45, and Metavir 46 systems. This is reasonable since both chronic viral hepatitis and AIH have a similar pattern of necro-inflammatory damage and share the morphogenesis of fibrosis. The choice of grading/staging system is arbitrary and depends on the pathologist’s preference and tradition, and on agreement with the local hepatologists. As in viral hepatitis, it is important to clearly specify the name of the used system in the final histological report, without which the scores lose their significance. Patients often consult different clinicians; therefore, this information is crucial for a proper interpretation of the histological report.
Grading and staging have prognostic and therapeutic implications and are required, since ALT values do not properly correlate with the disease severity and non-invasive tests for the assessment of fibrosis have not been fully validated in AIH 1.
According to EASL guidelines, treatment withdrawl is considered when biochemical remission is reached, but it requires histological remission, defined as normal histology or minimal hepatitis (Hepatitis Activity Index score/HAI < 4 or equivalent) 1.
Differential diagnosis
Since the pattern of injury and typical features of AIH are non-specific, the spectrum of histological differential diagnosis is very broad and an accurate integration with all clinical and laboratory features is required to reach a correct diagnosis.
VIRAL HEPATITIS
All acute and chronic viral hepatitis can appear similar to AIH and neither plasma cells nor the other histologic findings, which are more typically seen in AIH, are sensitive or specific enough. However, viral infection can be easily excluded by serological testing which are mandatory in all patients in whom a diagnosis of AIH is considered. A special attention should be paid to hepatitis E virus (HEV), since non-specific autoantibodies associated to AIH are frequently found during acute HEV infection. Clinicians and pathologists should be aware of this and always exclude HEV infection before diagnosing and treating AIH, as immunosuppression can lead to chronic HEV disease 47. A broad and highly variable spectrum, related to the clinical context, of histopathological findings can be observed in livers of patients infected with HEV, making the histopathological diagnosis very challenging 48. Immunohistochemistry for HEV pORF2 protein is a helpful histopathological tool 49.
DRUG INDUCED LIVER INJURY
Drug induced liver injury (DILI) represents the most challenging differential diagnosis, not only because it can mimic the clinical, biochemical, serological and morphological phenotype of AIH (AIH-like-DILI), but also because drugs may trigger latent or induce a de novo AIH 50-53. The distinction between DILI and AIH by histology can be extremely difficult (sometimes impossible), due to the absence of histological features pathognomonic of either DILI or AIH. Severe portal plasma cell-rich inflammation, prominent intralobular plasmacells and eosinophils, rosette formation, absence of cholestasis and presence of fibrosis have been suggested as features that are in favor of the diagnosis of AIH 54. The absence of cirrhosis, or advanced fibrosis, at presentation mainly suggests AIH-like DILI. A detailed clinical information is crucial, and the patient’s history should focus on recent exposure to drugs that can induce AIH-DILI. Fortunately, AIH-DILI usually responds to high doses of steroids as severe AIH usually does, but differently from true AIH that always relapses, steroid treatment can be discontinued without a DILI relapse 1.
PRIMARY BILIARY CHOLANGITIS
Differential diagnosis between AIH and classical PBC is not difficult. PBC is a chronic cholestatic syndrome showing a distinctive serological profile of positive antimitochondrial antibodies (AMAs), often associated with intense pruritus. Increase in serum IgM and alkaline phosphatase (ALP) levels with a normal or slightly elevated bilirubin level and only a mild elevation of ALT is typically observed at earlier stages. PBC may show moderate to severe portal inflammatory infiltrate with many plasma cells and even interface hepatitis, but prominent florid duct lesions and bile duct loss (not seen in AIH) are typically found in liver biopsies. Moreover, PBC does not show a hepatitic pattern of lobular injury, which is common in AIH. AMA-negative PBC may occur in about 10% of cases, but the histological scenario does not differ from the typical AMA-positive PBC. As already highlighted, stains for copper-associated protein and/or CK7 may be useful to recognize signs of chronic cholestasis in early stage disease 41.
Diagnosis of AIH-PBC overlap is discussed in another review in this special issue of Pathologica.
WILSON’S DISEASE
Wilson’s disease can show similar (or even identical) histologic findings to AIH and should always be considered among the differential diagnoses, particularly in younger patients. In most cases, it can be excluded based on serum ceruloplasmin levels and 24-hour urine copper. Rhodanin stain for copper detection in liver biopsy may be useful (bearing in mind that it can be negative even in Wilson disease, particularly in early stages) 55.
Conclusions
Liver biopsy is crucial for the establishment of a definite AIH diagnosis and it is recommended by the current guidelines at the time of presentation, to support the diagnosis and provide information about disease severity. Histology of AIH is typical in most cases (i.e. presence of interface hepatitis, plasma cell predominance in the portal inflammatory infiltrate, regenerative rosettes and emperipolesis), but it is considered in agreement with the possible AIH diagnosis when at least interface hepatitis is observed. Different conditions may mimic AIH at histology. Therefore, pathologists require all clinical and laboratory data, as well as imaging, which have to be provided by hepatologists. Once more, the most important aspect for an optimal patient management is communication between clinician and pathologist.
Figures and tables
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 2015;63:971-1004. https://doi.org/10.1016/j.jhep.2015.06.030. 10.1016/j.jhep.2015.06.030 Erratum in J Hepatol 2015;63:1543-1544. [DOI] [PubMed] [Google Scholar]
- 2.Assis DN. Immunopathogenesis of autoimmune hepatitis. Clin Liver Dis (Hoboken) 2020;15:129-132. https://doi.org/10.1002/cld.873 10.1002/cld.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka A. Autoimmune hepatitis: 2019 update. Gut Liver 2020;14:430-438. https://doi.org/10.5009/gnl19261 10.5009/gnl19261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeoman AD, Al-Chalabi T, Karani JB, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: implications for follow-up and screening. Hepatology 2008;48:863-870. https://doi.org/10.1002/hep.22432 10.1002/hep.22432 [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Gish R, Frederick T, et al. Development of hepatocellular carcinoma in autoimmune hepatitis patients: a case series. Dig Dis Sci 2011;56:578-585. https://doi.org/10.1007/s10620-010-1444-6 10.1007/s10620-010-1444-6 [DOI] [PubMed] [Google Scholar]
- 6.Czaja AJ. Diagnosis and management of autoimmune hepatitis. Clin Liver Dis 2015;19:57-79. https://doi.org/10.1016/j.cld.2014.09.004 10.1016/j.cld.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993;18:998-1005. https://doi.org/10.1002/hep.1840180435 10.1002/hep.1840180435 [DOI] [PubMed] [Google Scholar]
- 8.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-938. https://doi.org/10.1016/s0168-8278(99)80297-9 10.1016/s0168-8278(99)80297-9 [DOI] [PubMed] [Google Scholar]
- 9.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169-176. https://doi.org/10.1002/hep.22322 10.1002/hep.22322 [DOI] [PubMed] [Google Scholar]
- 10.Zachou K, Muratori P, Koukoulis GK, et al. Review article: autoimmune hepatitis - Current management and challenges. Aliment Pharmacol Ther 2013;38:887-913. https://doi.org/10.1111/apt.12470 10.1111/apt.12470 [DOI] [PubMed] [Google Scholar]
- 11.Muratori P, Granito A, Quarneti C, et al. Autoimmune hepatitis in Italy: the Bologna experience. J Hepatol 2009;50:1210-1218. https://doi.org/10.1016/j.jhep.2009.01.020 10.1016/j.jhep.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 12.Al-Chalabi T, Underhill JA, Portmann BC, et al. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol 2008;48:140-147. https://doi.org/10.1016/j.jhep.2007.08.013 10.1016/j.jhep.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 13.Floreani A, Niro G, Rosa Rizzotto E, et al. Type I autoimmune hepatitis: clinical course and outcome in an Italian multicenter study. Aliment Pharmacol Ther 2006;24:1051-1057. https://doi.org/10.1111/j.1365-2036.2006.03104.x 10.1111/j.1365-2036.2006.03104.x [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara K, Fukuda Y, Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute onset autoimmune hepatitis. J Gastroenterol 2008;43:951-958. https://doi.org/10.1007/s00535-008-2254-x 10.1007/s00535-008-2254-x [DOI] [PubMed] [Google Scholar]
- 15.Yasui S, Fujiwara K, Yonemitsu Y, et al. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol 2011;46:378-390. https://doi.org/10.1007/s00535-010-0316-3 10.1007/s00535-010-0316-3 [DOI] [PubMed] [Google Scholar]
- 16.Czaja AJ. Autoantibody-negative autoimmune hepatitis. Dig Dis Sci 2012;57:610-624. https://doi.org/10.1007/s10620-011-2017-z 10.1007/s10620-011-2017-z [DOI] [PubMed] [Google Scholar]
- 17.Zeman MV, Hirschfield GM. Autoantibodies and liver disease: uses and abuses. Can J Gastroenterol 2010;24:225-231. doi:10.1155/2010/431913 10.1155/2010/431913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijeyesinghe U, Russell AS. Outcome of high titer antinuclear antibody positivity in individuals without connective tissue disease: a 10-year follow-up. Clin Rheumatol 2008;27:1399-402. https://doi.org/10.1007/s10067-008-0932-y 10.1007/s10067-008-0932-y [DOI] [PubMed] [Google Scholar]
- 19.Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000;124:71-81. https://doi.org/10.1043/0003-9985(2000)124<0071:GFCUOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 20.Cassani F, Cataleta M, Valentini P, et al. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology 1997;26:561-6. https://doi.org/10.1002/hep.510260305 10.1002/hep.510260305 [DOI] [PubMed] [Google Scholar]
- 21.Vuppalanchi R, Gould RJ, Wilson LA, et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int 2012;6:379-385. https://doi.org/10.1007/s12072-011-9277-8 10.1007/s12072-011-9277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: Does it exist? A published work review. Hepatol Res 2011;41:498-504. https://doi.org/10.1111/j.1872-034X.2011.00808.x 10.1111/j.1872-034X.2011.00808.x [DOI] [PubMed] [Google Scholar]
- 23.Miyake Y, Iwasaki Y, Terada R, et al. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther 2006;23:1347-1353. https://doi.org/10.1111/j.1365-2036.2006.02894.x 10.1111/j.1365-2036.2006.02894.x [DOI] [PubMed] [Google Scholar]
- 24.Abe M, Mashiba T, Zeniya M, et al. Present status of autoimmune hepatitis in Japan: a nationwide survey. J Gastroenterol 2011;46:1136-1141. https://doi.org/10.1007/s00535-011-0421-y 10.1007/s00535-011-0421-y [DOI] [PubMed] [Google Scholar]
- 25.Lohse AW, Mieli-Vergani G. Autoimmune hepatitis. J Hepatol 2011;55:171-182. https://doi.org/10.1016/j.jhep.2010.12.012 10.1016/j.jhep.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 26.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193-2213. https://doi.org/10.1002/hep.23584 10.1002/hep.23584 [DOI] [PubMed] [Google Scholar]
- 27.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 2011;60:1611-1629. https://doi.org/10.1136/gut.2010.235259 10.1136/gut.2010.235259 [DOI] [PubMed] [Google Scholar]
- 28.Czaja AJ. Autoimmune hepatitis in special patient populations. Best Pract Res Clin Gastroenterol 2011;25:689-700. https://doi.org/10.1016/j.bpg.2011.09.011 10.1016/j.bpg.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 29.Tiniakos DG, Brain JG, Bury YA. Role of histopathology in autoimmune hepatitis. Dig Dis. 2015;33 Suppl 2:53-64. https://doi.org/10.1159/000440747 10.1159/000440747 [DOI] [PubMed] [Google Scholar]
- 30.Guindi M. Histology of autoimmune hepatitis and its variants. Clin Liver Dis 2010;14: 577-590. https://doi.org/10.1016/j.cld.2010.07.003 10.1016/j.cld.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 31.Gurung A, Assis DN, McCarty TR, et al. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol 2018;82:51-60. https://doi.org/10.1016/j.humpath.2018.07.014 10.1016/j.humpath.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 32.Rubio CA, Truskaite K, Rajani R, et al. A method to assess the distribution and frequency of plasma cells and plasma cell precursors in autoimmune hepatitis. Anticancer Res 2013;33:665-669. [PubMed] [Google Scholar]
- 33.Washington MK, Manns MP. Autoimmune hepatitis. Burt AD, Portmann B, Ferrel L, eds. MacSween’s Pathology of the Liver. Edinburgh: Churchill Livingstone. 2012, pp 467-490. [Google Scholar]
- 34.Verdonk RC, Lozano MF, van den Berg AP, et al. Bile ductal injury and ductular reaction are frequent phenomena with different significance in autoimmune hepatitis. Liver Int 2016. September;36(9):1362-1369. https://doi.org/10.1111/liv.13083 10.1111/liv.13083 [DOI] [PubMed] [Google Scholar]
- 35.Okano N, Yamamoto K, Sakaguchi K, et al. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol Res 2003;25:263-270. https://doi.org/10.1016/s1386-6346(02)00274-7 10.1016/s1386-6346(02)00274-7 [DOI] [PubMed] [Google Scholar]
- 36.Hofer H, Oesterreicher C, Wrba F, et al. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 2006;59:246-249. https://doi.org/10.1136/jcp.2005.029348 10.1136/jcp.2005.029348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer YS, van Nieuwkerk CM, Witte BI, et al. Assessment of the histopathological key features in autoimmune hepatitis. Histopathology 2015;66:351-362. https://doi.org/10.1111/his.12558 10.1111/his.12558 [DOI] [PubMed] [Google Scholar]
- 38.Miao Q, Bian Z, Tang R, et al. Emperipolesis mediated by CD8 T cells is a characteristic histopathologic feature of autoimmune hepatitis. Clin Rev Allergy Immunol 2015;48(2-3):226-235. https://doi.org/10.1007/s12016-014-8432-0 10.1007/s12016-014-8432-0 [DOI] [PubMed] [Google Scholar]
- 39.Czaja AJ, Carpenter HA. Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology 2001;34(4 Pt 1):659-665. https://doi.org/10.1053/jhep.2001.27562 10.1053/jhep.2001.27562 [DOI] [PubMed] [Google Scholar]
- 40.Zen Y, Harada K, Sasaki M, et al. Are bile duct lesions of primary biliary cirrhosis distinguishable from those of autoimmune hepatitis and chronic viral hepatitis? Interobserver histological agreement on trimmed bile ducts. J Gastroenterol 2005;40:164-170. https://doi.org/10.1007/s00535-004-1514-7 10.1007/s00535-004-1514-7 [DOI] [PubMed] [Google Scholar]
- 41.Balitzer D, Shafizadeh N, Peters MG, et al. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol 2017;30:773-783. https://doi.org/10.1038/modpathol.2016.267 10.1038/modpathol.2016.267 [DOI] [PubMed] [Google Scholar]
- 42.Bihari C, Rastogi A, Sarin SK. Postinfantile giant cell hepatitis: an etiological and prognostic perspective. Hepat Res Treat 2013;2013:601290. https://doi.org/10.1155/2013/601290 10.1155/2013/601290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker SM, Jonas MM, Perez-Atayde AR. Hyaline droplets in Kupffer cells: a novel diagnostic clue for autoimmune hepatitis. Am J Surg Pathol 2015;39:772-778. https://doi.org/10.1097/PAS.0000000000000395 10.1097/PAS.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 44.Scheuer PJ: Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991;13: 372-374. https://doi.org/10.1016/0168-8278(91)90084-o 10.1016/0168-8278(91)90084-o [DOI] [PubMed] [Google Scholar]
- 45.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-699. https://doi.org/10.1016/0168-8278(95)80226-6 10.1016/0168-8278(95)80226-6 [DOI] [PubMed] [Google Scholar]
- 46.The French METAVIR cooperative study group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15-20. [PubMed] [Google Scholar]
- 47.Fujiwara S, Yokokawa Y, Morino K, et al. Chronic hepatitis E: a review of the literature. J Viral Hepat 2014;21:78-89. https://doi.org/10.1111/jvh.12156 10.1111/jvh.12156 [DOI] [PubMed] [Google Scholar]
- 48.Lenggenhager D, Pawel S, Honcharova-Biletska H, et al. The histologic presentation of hepatitis E reflects patients’ immune status and pre-existing liver condition. Mod Pathol 2021;34:233-248. https://doi.org/10.1038/s41379-020-0593-1 10.1038/s41379-020-0593-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenggenhager D, Weber A. Clinicopathologic features and pathologic diagnosis of hepatitis E. Hum Pathol 2020;96:34-38. https://doi.org/10.1016/j.humpath.2019.10.003 10.1016/j.humpath.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 50.Kleiner DE. Recent Advances in the histopathology of drug-induced liver injury. Surg Pathol Clin 2018;11:297-311. https://doi.org/10.1016/j.path.2018.02.009 10.1016/j.path.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Buey L, Garcia-Monzon C, Rodriguez S, et al. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology 1995;108:1770-1777. https://doi.org/10.1016/0016-5085(95)90139-6 10.1016/0016-5085(95)90139-6 [DOI] [PubMed] [Google Scholar]
- 52.Takahashi A, Kanno Y, Takahashi Y, et al. Development of autoimmune hepatitis type 1 after pulsed methylprednisolone therapy for multiple sclerosis: a case report. World J Gastroenterol 2008;14:5474-5477. https://doi.org/10.3748/wjg.14.5474 10.3748/wjg.14.5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleiner DE. Histopathological challenges in suspected drug-induced liver injury. Liver Int 2018. 38:198-209. https://doi.org/10.1111/liv.13584 10.1111/liv.13584 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology 2011;54:931-939. https://doi.org/10.1002/hep.24481 10.1002/hep.24481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schilsky ML. Wilson disease: genetic basis of copper toxicity and natural history. Semin Liver Dis 1996;16:83-95. https://doi.org/10.1055/s-2007-1007221 10.1055/s-2007-1007221 [DOI] [PubMed] [Google Scholar]


