Abstract
Background
Optimal antithrombotic management of patients with pre-existing atrial fibrillation (AF) undergoing transcatheter aortic valve replacement (TAVR) is challenging given the need to balance the risk of bleeding and thromboembolism. We aimed to examine variation in care and association of antithrombotic therapies with 1-year outcomes of stroke, bleeding and mortality in patients undergoing TAVR with concomitant AF in the US.
Methods
Patients who underwent TAVR with pre-existing AF from November, 2011 through September, 2015 in the STS/ACC TVT® registry linked to the Medicare database were examined according to receipt of oral anticoagulants (OAC) or antiplatelet therapies (APT) or a combination of these (OAC+APT) at discharge. To assess the associations of antithrombotic therapies with 1-year outcomes of stroke, bleeding, and mortality; we utilized inverse probability weighting for antithrombotic therapies and multivariable regression modeling to adjust for patient- and hospital-level variables.
Results
In the 11382 patients included in our study, 5833 (51.2%) were discharged on OAC+APT, 4786 (42.0%) on APT alone and 763 (6.7%) on OAC alone. There was significant variability in discharge medication patterns, including 42% of patients discharged without OAC therapy. In adjusted analyses, the risk for all-cause mortality and stroke was not significantly different when comparing the three different antithrombotic strategies. Risk of bleeding was higher with OAC+APT compared with APT alone (HR 1.16, 95% CI 1.05–1.27) and similar compared with OAC alone (HR 1.17, 95% CI 0.93–1.47).
Conclusions
There was significant variability in discharge medication patterns across U.S. sites in patients with AF undergoing TAVR, including significant underuse of OAC in this high risk cohort. The use of OAC+APT (vs. OAC alone or APT alone) was not associated with a lower risk of stroke or mortality but was associated with increased risk of bleeding complications at 1-year compared to APT alone.
Keywords: aortic valve replacement, atrial fibrillation, anticoagulation, transcatheter aortic valve implantation, stroke, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Anticoagulants, Ischemic Stroke
Introduction
Atrial fibrillation is a common problem among patients undergoing cardiovascular procedures, including TAVR, and presents a challenging problem for clinicians as two of the most frequent complications after transcatheter aortic valve replacement (TAVR) include stroke and bleeding.1 In addition, several studies have demonstrated the occurrence of prosthetic valve thrombosis after TAVR with an incidence ranging from 7% to 20%.2, 3 To minimize the risk for stroke, antiplatelet or anticoagulant therapies are commonly prescribed after TAVR for varying durations of time, leading to an increased risk of bleeding. However striking the balance between preventing stroke without causing excessive bleeding is difficult in high risk populations such as those patients with AF undergoing TAVR.4 Despite the possibility of exponentially higher bleeding risk, addition of an oral anticoagulant agent to the antiplatelet regimen becomes necessary in the setting of increased thromboembolic risk from AF.
To date, evidence-based guidance on the optimal antithrombotic regimen is lacking for patients with AF undergoing TAVR. A single randomized trial has been published evaluating the use of anticoagulation with or without antiplatelet therapy for patients with AF undergoing TAVR5, however it was likely underpowered to detect differences in ischemic stroke outcomes. As such, none of the major international societies including European guidelines6, Canadian guidelines7 and US guidelines8 adequately address the challenges surrounding antithrombotic therapy in patients with concomitant AF undergoing TAVR. There is also evidence of variability in prescribing patterns for patients with AF undergoing PCI9, 10, and evidence of underuse of OAC in patients with AF across the world11, despite ample data to suggest its benefits. In addition, there is no data exploring the use of antithrombotic agents in TAVR patients with pre-existing AF in the real world setting across the United States. Thus we examined the Society of Thoracic Surgeons/ American College of Cardiology (STS/ACC) - Transcatheter Valve Therapy (TVT) Registry through the National Cardiovascular Data Registry (NCDR) program, a nationally representative sample for patients undergoing TAVR, to determine current treatment patterns for antithrombotic therapies at the time of discharge in patients with pre-existing AF in the United States. Using the linkage of the STS/TVT registry to the Centers for Medicare and Medicaid Services (CMS), we also evaluated the relationship between these therapies and clinical outcomes up to 1 year after TAVR.
Methods
All data and materials are property of the Society of Thoracic Surgeons (STS), American College of Cardiology (ACC) Transcatheter Valve Therapies (TVT) Registry which is governed in part by the NCDR. The authors do not have authority to grant access to all available data in the registry. All inquiries for data should be made directly to the STS/ACC TVT Registry.
Data
The NCDR STS/ACC TVT Registry is a joint initiative between stakeholders in professional societies, government, and industry. The Society of Thoracic Surgeons and American College of Cardiology provide leadership to the registry through the NCDR program, and the Duke Clinical Research Institute serves as the center for housing and analysis of the data. Participating centers use standardized definitions to collect information on consecutive TAVR cases. The NCDR warehouse and the Duke Clinical Research Institute Data Analysis Center both implement data quality checks, including feedback reports, and examine data ranges and consistency to optimize completeness and accuracy. The data quality is randomly audited with evidence of >85% accuracy.12 For protection of patient participants, the STS and ACC have signed a Federal Wide Assurance with the Department of Health and Human Services, and all research is conducted in compliance with the Common Rule (45 CFR§46). A central institutional review board (Chesapeake Research Review Inc.) approves activities of the TVT registry. The present investigation has been granted a waiver of informed consent.
Study Population
All patients who underwent TAVR from November 9, 2011 through September 30, 2015 in the TVT registry linked to the Centers of Medicare and Medicaid Services (CMS) were included in our initial cohort of 32,882 patients (Figure 1). We excluded 1186 patients who had in-hospital death (and thus may not have received antithrombotic medications), 275 patients with missing data on discharge medications, 64 patients with unknown history of atrial fibrillation or flutter, 1841 patients who developed atrial fibrillation during hospitalization, 2827 patients who were censored before discharge (due to end of CMS follow-up or Medicare part A&B eligibility), 553 patients who were discharged without any antithrombotic therapies and 66 patients discharged on multiple anticoagulants leading to a cohort of 26070 patients from 387 hospitals in the US. We then excluded 14688 patients without history of atrial fibrillation or flutter prior to the TAVR procedure, leading to a final cohort of 11382 patients.
Figure 1.
Study Sample Selection
Outcomes
We examined the baseline characteristics of patients according to receipt of oral anticoagulants (OAC) or antiplatelet therapies (APT) or a combination of these (OAC+APT) at discharge. We also evaluated outcomes including stroke, all-cause mortality, and bleeding events at 1 year of follow-up. Patients were censored at 1 year after discharge or end of CMS follow-up (10/1/2015), whichever occurred first, for the analysis of all-cause mortality. For the analyses of stroke and bleeding, patients were censored at 1 year after discharge, end of CMS follow-up (10/1/2015), or end of Medicare part A & B Fee-for-Service eligibility, whichever occurred first. We utilized CMS-linked claims data to identify hospitalizations for stroke using ICD-9 codes (433.x1, 434.x1, 997.02, 436, 437.1, 437.9, 430, 431, 432.x). Similarly, we identified bleeding using ICD-9 codes for gastrointestinal, genitourinary, intracranial, critical, access-site or other bleeding.
Statistical Methods
Baseline clinical characteristics were compared between patients who were discharged with OAC alone vs. APT alone vs. OAC+APT. Continuous variables were summarized as medians and interquartile ranges (IQRs) and compared using the Kruskal-Wallis test; categorical variables were summarized as counts and percentages and compared using the Pearson chi-square test or Fisher’s exact test. Cumulative incidence of outcomes at 1 year (all-cause mortality, stroke and bleeding) was compared among patients with concomitant AF discharged with OAC alone vs. APT alone vs. OAC+APT using Gray’s method. Cumulative incidence of mortality was estimated using the Kaplan-Meier method. For nonfatal outcomes, death was considered as a competing risk.
To assess the associations of discharge antithrombotic therapies with 1-year outcomes in patients with concomitant AF, we first constructed a multivariable multinomial logistic regression model to predict the propensity of receiving one of the antithrombotic therapies (OAC alone, APT alone, or OAC+APT) for each patient. When modeling the outcomes, patients were weighed based on their propensity using the inverse probability weighting method. Variables used in the propensity model included patient- and hospital-level variables, as described in a recent publication on antithrombotic use after surgical bioprosthetic valve replacement13 : age, sex, sex-specific body surface area, left ventricular ejection fraction, hemoglobin, platelet count, glomerular filtration rate, number of days from 11/1/2011 until procedure date, race (non-Hispanic white vs. other), current dialysis, left main stenosis ≥50%, proximal LAD ≥ 70%, prior myocardial infarction, endocarditis, prior stroke or transient ischemic attack, carotid stenosis, prior peripheral arterial disease, current/recent smoker, diabetes, New York Heart Association (NYHA) class IV, severe chronic lung disease, home oxygen, hostile chest, porcelain aorta, access site (femoral vs. other), pacemaker, previous implantable cardioverter defibrillator, prior percutaneous coronary implantation, prior coronary artery bypass grafting, prior cardiac operations (2+ vs. 1 vs. 0), prior aortic valve procedure, prior non-aortic valve procedure, aortic etiology (degenerative vs. other), valve morphology (tricuspid vs. other), moderate/severe aortic insufficiency, moderate/severe mitral insufficiency, moderate/severe tricuspid insufficiency, acuity of TAVR (elective vs. urgent vs. shock or inotropes or assist device vs. emergency or salvage or cardiac arrest), hospital annual TAVR volume, hospital region (Northeast vs. West vs. Midwest vs. South), hospital ownership (private/community vs. university vs. government), and metropolitan area (urban vs. suburban vs. rural).
The associations of antithrombotic therapies with mortality were assessed using Cox proportional hazards models. Nonfatal outcomes (stroke and bleeding) were assessed using the Fine and Gray’s proportional sub-distribution hazards models with death being a competing risk. Clustering of patients within hospitals was taken into account using a robust variance estimation method. For each outcome, two models were built, 1) unadjusted model including antithrombotic therapies only, and 2) adjusted model including antithrombotic therapies and covariates listed above. Missing data on the covariates were handled with single imputation, i.e., missing values were imputed to median for continuous variables and to mode for categorical variables. All covariates in the adjusted models had a missing rate of ≤ 2% except that carotid stenosis had a missing rate of 16%. The results were presented as hazard ratios (HRs) with 95% confidence intervals (CI). A two-sided p-value of <0.05 was considered statistically significant.
All statistical analyses data were performed by the Duke Clinical Research Institute using SAS software (version 9.4, SAS Institute Inc., Cary, NC).
Results
We utilized CMS-linked TVT registry data from November 2011 through September 2015 for our study. Out of 26,070 patients at 387 hospitals who underwent TAVR during the study period, 11382 patients (43.7%) had history of atrial fibrillation and were included in our final study sample (Figure 1).
Patient Characteristics
Out of the 11382 patients included in our study, 5833 (51.2%) were discharged on OAC+APT therapy, 4786 (42.0%) on APT alone and 763 (6.7%) on OAC alone. Baseline characteristics according to prescription at discharge of OAC alone versus APT alone versus OAC+APT are shown in Table 1. When compared with patients who received OAC alone or OAC+APT, patients discharged with APT alone were less likely to be women, have prior stroke, and femoral access for their TAVR; and more likely to have coronary artery disease, prior aortic valve procedure, dialysis-dependent renal failure and higher STS PROM score. Estimated stroke and bleeding risk, calculated as median CHADS2VAsc2 and ATRIA scores, were numerically similar between these groups though there may have been small, likely clinically insignificant differences (Table 1).
Table 1.
Baseline characteristics of patients with AF undergoing TAVR discharged on OAC alone, APT alone, or OAC+APT therapy
| OAC Alone N=763 | APT Alone N=4786 | OAC+APT N=5833 | p-value | |
|---|---|---|---|---|
| Age, years | 85.0 (79.0–88.0) | 85.0 (79.0–89.0) | 84.0 (79.0–88.0) | <.001 |
| Female | 406 (53.2%) | 2,100 (43.9%) | 2,676 (45.9%) | <.001 |
| White Race | 734 (96.8%) | 4,628 (96.9%) | 5,699 (98.1%) | <.001 |
| STS PROM Score, % | 7.4 (5.2–11.1) | 8.3 (5.6–12.3) | 7.5 (5.2–11.2) | <.001 |
| CHADS2-Vasc Score, median (IQR) | 5.0 (4.0–6.0) | 5.0 (5.0–6.0) | 5.0 (5.0–6.0) | 0.007 |
| ATRIA Score, median (IQR) | 6.0 (3.0–6.0) | 6.0 (3.0–6.0) | 6.0 (3.0–6.0) | <.001 |
| NYHA Class III/IV within 2 Weeks | 624 (82.4%) | 3,956 (83.6%) | 4,911 (84.9%) | 0.075 |
| Coronary Artery Disease | 302 (39.7%) | 2,953 (61.8%) | 3,284 (56.4%) | <.001 |
| Prior Open Heart Surgery | 223 (29.2%) | 1,654 (34.6%) | 1,992 (34.2%) | 0.013 |
| Prior Aortic Valve Procedure | 107 (14.0%) | 851 (17.8%) | 836 (14.3%) | <.001 |
| Prior Stroke | 119 (15.6%) | 604 (12.6%) | 849 (14.6%) | 0.005 |
| Peripheral Arterial Disease | 205 (27.0%) | 1,498 (31.4%) | 1,788 (30.7%) | 0.053 |
| Severe COPD | 89 (11.8%) | 696 (14.7%) | 882 (15.2%) | 0.041 |
| Oxygen-Dependent Lung Disease | 92 (12.1%) | 697 (14.6%) | 773 (13.3%) | 0.056 |
| Current Dialysis | 20 (2.6%) | 229 (4.8%) | 158 (2.7%) | <.001 |
| Permanent Pacemaker/ICD | 238 (31.2%) | 1,294 (27.0%) | 1,833 (31.4%) | <.001 |
| Hostile Chest | 53 (7.0%) | 344 (7.2%) | 427 (7.3%) | 0.920 |
| Porcelain Aorta | 45 (5.9%) | 233 (4.9%) | 348 (6.0%) | 0.043 |
| LVEF < 30% | 56 (7.5%) | 362 (7.7%) | 495 (8.6%) | 0.199 |
| Femoral Access | 592 (78.5%) | 3,466 (72.7%) | 4,332 (74.6%) | 0.002 |
STS PROM = Society of Thoracic Surgeons Predicted Risk of Mortality at 30 days; CHADs2- VASC = congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65 to 74 years, sex category; ATRIA = Anticoagulation and Risk Factors in Atrial Fibrillation; NYHA = New York Heart Association Functional Class;
Variation in Use of Antiplatelet agents and OAC therapies
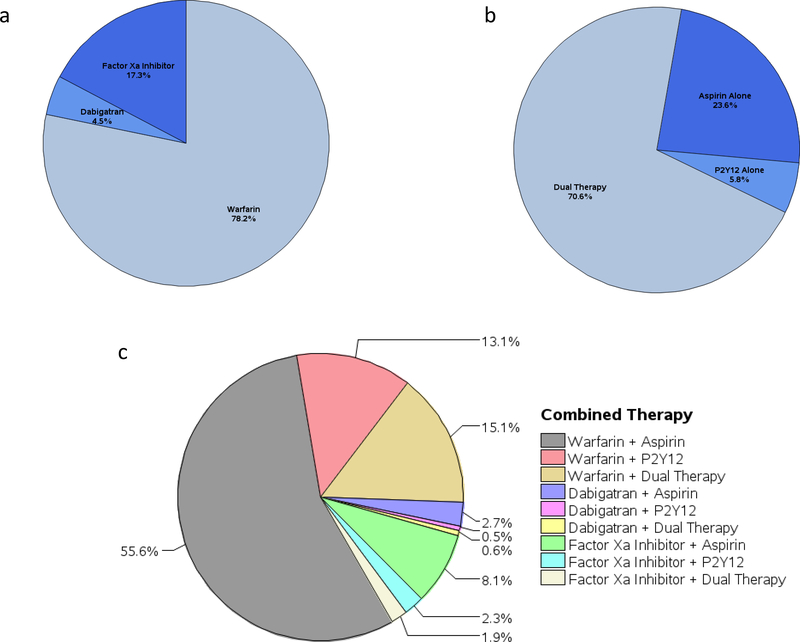
Among the 4786 patients discharged on APT alone, about 70.6% were prescribed dual antiplatelet with aspirin and a P2Y12 inhibitor, 23.6% aspirin alone and 5.8% P2Y12 inhibitor alone. Similarly, out of 763 patients discharged on OAC alone, the majority (78.2%) were prescribed warfarin, 17.3% received a factor Xa inhibitor and 4.5% received dabigatran. Finally, out of 5833 patients discharged on OAC+APT therapy, the most common combination was that of warfarin and aspirin in 55.6% of the patients, followed by warfarin and dual APT (15.1%) and warfarin and P2Y12 inhibitor (13.1%). Dabigatran and factor Xa inhibitors were prescribed in combination with APT less frequently (Figure 2). Triple therapy (OAC and dual APT) was prescribed at discharge in only 17.6% of the total study sample.
Figure 2.
a. Proportion of different anticoagulant therapies at discharge among patients who received OAC alone. b. Proportion of different antiplatelet therapies at discharge among patients who received APT alone. c. Proportion of different antiplatelet and anticoagulant combination therapies at discharge among patients who received OAC+APT
Clinical Outcomes
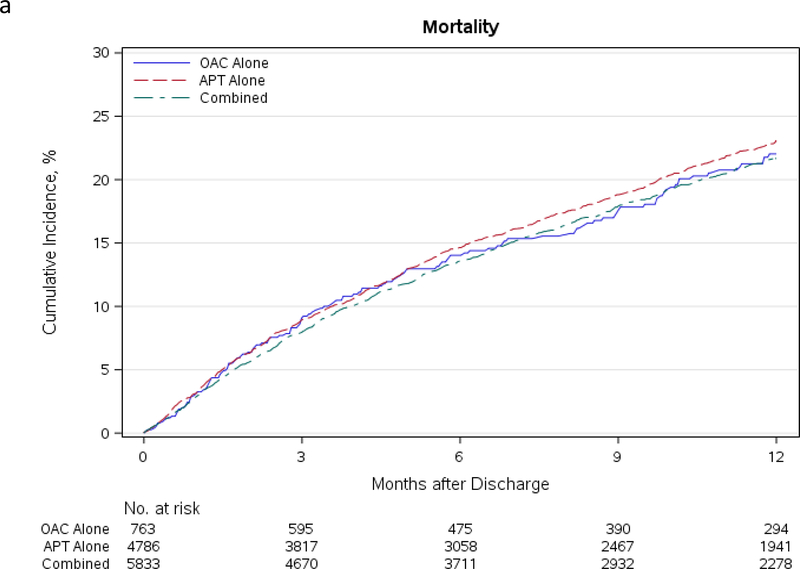
In unadjusted analyses, there were no differences in the cumulative incidence of mortality at 1 year for patients discharged on the three different antithrombotic regimens (OAC alone 22.0%, 95% CI: 18.8% - 25.8%; APT alone 23.1%, 95% CI: 21.7% - 24.5%; OAC+APT 21.7%, 95% CI: 20.5% - 23.0%). Rates of stroke were similarly low at 1 year in all three groups (OAC alone 1.3%, 95% CI: 0.6% - 2.6%; APT alone 2.5%, 95% CI: 2.0% - 3.1%; OAC+APT 2.3%, 95% CI: 1.9% - 2.8%). Bleeding rates were the highest in patients receiving OAC+APT (21.1%, 95% CI: 19.9% - 22.3%), followed by APT alone (19.5%, 95% CI: 18.3% - 20.8%) and OAC alone (17.6%, 95% CI: 14.7% - 21.0%). The cumulative incidence curves for these outcomes at 1-year are shown in Figure 3.
Figure 3.
a. Cumulative incidence of mortality in AF patients discharged on OAC alone, APT alone, and OAC+APT. b. Cumulative incidence of stroke in AF patients discharged on OAC alone, APT alone, and OAC+APT therapy. c. Cumulative incidence of bleeding in AF patients discharged on OAC alone, APT alone, and OAC+APT
In the risk-adjusted analyses with inverse probability weighting, the hazard ratios for all-cause mortality and stroke were similar when comparing the three different antithrombotic strategies (Table 2). Bleeding, however, was much less likely to occur in patients receiving APT alone when compared with those on OAC+APT therapy (HR 0.85, 95% CI 0.78 – 0.94). Risk of bleeding was similar when comparing APT alone with OAC alone (HR 0.99, 95% CI 0.79 – 1.24) or OAC+APT with OAC alone (HR 1.16, 95% CI 0.93–1.44).
Table 2:
Associations between antithrombotic therapies and 1-year outcomes in patients with AF
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Outcome | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Mortality | APT alone vs. OAC alone | 0.90 (0.72–1.11) | 0.31 | 0.88 (0.71–1.09) | 0.25 |
| OAC+APT vs. OAC alone | 0.90 (0.74–1.11) | 0.34 | 0.90 (0.73–1.12) | 0.34 | |
| OAC+APT vs. APT alone | 1.01 (0.92–1.10) | 0.83 | 1.03 (0.93–1.12) | 0.57 | |
| Stroke | APT alone vs. OAC alone | 1.47 (0.59–3.68) | 0.41 | 1.51 (0.59–3.89) | 0.39 |
| OAC+APT vs. OAC alone | 1.21 (0.48–3.05) | 0.68 | 1.24 (0.48–3.20) | 0.65 | |
| APT alone vs. OAC+APT | 1.21 (0.93–1.58) | 0.15 | 1.22 (0.93–1.59) | 0.15 | |
| Bleeding | APT alone vs. OAC alone | 1.01 (0.80–1.27) | 0.94 | 0.99 (0.79–1.24) | 0.93 |
| OAC+APT vs. OAC alone | 1.17 (0.93–1.47) | 0.17 | 1.16 (0.93–1.44) | 0.19 | |
| OAC+APT vs. APT alone | 1.16 (1.05–1.27) | 0.002 | 1.17 (1.06–1.28 | 0.001 | |
Adjusted and Unadjusted Hazard ratios are described here for different AT therapy combinations in comparison to the referent (listed last) for Mortality, Stroke, and Bleeding at 1 year. Adjusted hazard ratios were calculated using the aforementioned propensity model with previously described variables.
Discussion
This analysis is the first national, multicenter, real-world study from the United States evaluating variation of antithrombotic regimens prescribed at discharge and their association with the occurrence of both thrombotic and bleeding-related events in patients with AF undergoing TAVR. Over 40% of the patients who underwent TAVR in this study had concomitant AF, posing a therapeutic challenge balancing the risk of bleeding and thrombotic complications. We found substantial heterogeneity in prescription patterns of different antithrombotic regimens at discharge highlighting the lack of consensus on optimal therapy for this population. Also, at least 42% of these patients were not prescribed an OAC at discharge, despite an absolute indication for it. Finally, counter to the hypothetical superiority of combined OAC and APT therapy for preventing thromboembolic complications, we found that combined therapy did not confer benefit when compared with OAC alone and showed an increased risk of bleeding when compared with the APT alone strategy. Further clinical investigation is warranted to determine the optimal antithrombotic therapy in this high-risk population. Our large scale, nationally representative, observational study is timely and adds to the scientific literature in several important ways.
Our analysis provides new information about heterogeneity of antithrombotic regimens prescribed at discharge in the TAVR population. There were over a dozen different combinations of OAC and APT used at discharge for these patients. Among patients undergoing TAVR who had an indication for chronic OAC due to AF, 51% were discharged on combination OAC and APT while 42% were discharged without OAC. Our findings differ from the study by Altisent et al.14, among whom the majority of the patients (84%) were discharged on OAC+APT. This further highlights the heterogeneity in practice patterns internationally and may be reflective of differences region-specific guidelines and practice styles. There remains no consensus among contemporary guidelines from several international specialty societies regarding optimal antithrombotic strategies for these patients who have augmented risk of both thromboembolic and bleeding complications. American-based guidelines recommend warfarin, a direct thrombin inhibitor, or a factor Xa inhibitor in association with low-dose aspirin based on expert consensus15, while the European guidelines recommend prescription of vitamin K antagonist in addition to aspirin or thienopyridine.6 In contrast, the Canadian position statement states a direct acting OAC should be used when possible added to aspirin and that triple therapy is discouraged.7 Such incongruity of guidance may be confusing to practitioners and is secondary to lack of sufficient evidence to guide management. Fortunately, there are several ongoing randomized controlled trials to investigate optimal regimens.
The underuse of OAC in high risk patients with AF, such as those who undergo TAVR, is an important finding. Our patient population was high risk with a median CHADS2-VASC score of 5 and 75% of patients with CHADS2-VASC ≥ 4, thus with an absolute indication for OAC. Concomitant CAD/PCI or other indications for dual antiplatelet therapy can make these decisions difficult, but this represented a minority of patients in our study. OAC may be underused in many patients with AF due to concerns for bleeding risk or underestimation of stroke risk.11 Previous investigation has shown that physicians do not accurately assess bleeding risk in patients with AF which may lead to unnecessary avoidance16. Multiple groups have undertaken strategies to improve adherence to guideline recommended therapies for AF patients at moderate to high risk for stroke.17, 18 Patients with AF undergoing TAVR represent a very high risk cohort and stroke remains a common and potentially devastating complication of TAVR. Ongoing trials such as AVATAR (Anticoagulation Alone Versus Anticoagulation and Aspirin Following Transcatheter Aortic Valve Interventions (1:1), NCT02735902), ENVISAGE-TAVI AF (Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation, NCT02943785), and ATLANTIS (Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis, NCT02664649) are specifically designed to explore the safety and efficacy of either novel OACs or vitamin K antagonists in patients with AF undergoing TAVR. These data may help clinicians to further understand the use of anticoagulation after TAVR and perhaps emphasize the importance of OAC in these high risk patients.
We also found that despite an intuitive mechanistic benefit for preventing thromboembolic complications, use of OAC+APT did not translate into a reduced risk of stroke or all-cause mortality compared with either OAC alone or APT alone, while bleeding risk was increased compared with APT alone and similar compared with OAC alone. While the size and scope of our study allowed us to draw national-level inferences about the thrombotic and bleeding effects of these different strategies, our findings are in agreement with a small multicenter study of 621 patients by Altisent et al.14 that also seemed to discredit the hypothetical superiority of OAC+APT therapy whilst increasing risk of bleeding. The recently published POPULAR-TAVI trial randomized patients with AF undergoing TAVR to OAC alone vs. OAC with clopidogrel in 326 patients.5 The results indicated potential benefit for OAC therapy alone with significantly lower rates of major bleeding (21.7% vs. 34.6%, risk ratio, 0.63; 95% CI 0.43 to 0.90; P=0.01) and no significant difference in ischemic events compared with OAC plus clopidogrel. Our results were similar, indicating combined OAC + APT therapy increased risk of bleeding when compared with the APT alone strategy. Though we saw a similar elevated hazard ratio for bleeding with OAC +APT therapy compared with OAC alone, the confidence intervals were broader due to the small sample of patients with OAC alone. We also found that use of OAC+APT did not translate into a reduced risk of stroke or all-cause mortality compared with either OAC alone or APT alone. This is in agreement with the aforementioned POPULAR TAVI trial5 and the study by Altisent et al.14 Our findings are also consistent with a recently published analysis from the PARTNER II trial and associated registries in which the authors reported no difference in the combined endpoint of death or stroke at 2 years for patients receiving OAC alone, APT alone or OAC+APT.19 These findings also mirror those from other populations with AF who may have other indications for APT, including those with coronary artery disease. A prospective observational study of 8,700 patients with AF and stable coronary disease showed that the addition of APT to OAC was not associated with the reduction of recurrent coronary events or stroke compared with OAC therapy alone, yet increased the risk of bleeding events.20 Patients with AF who have acute coronary syndromes or receive percutaneous coronary intervention represent a higher risk group for whom current evidence supports use of combined OAC and single APT.21 For patients with AF undergoing surgical aortic valve replacement, there are no prospective studies comparing different antithrombotic strategies and guidelines are similarly empirically based.
An optimal regimen that minimizes risk of bleeding without increasing thromboembolic complications would be highly desirable, not only in terms of reduced morbidity and mortality, but also in reduced costs to the healthcare system. In an analysis from the Placement of Aortic Transcatheter Valve (PARTNER) I trial, the adjusted incremental cost of a major bleeding event after TAVR was estimated at 35823 USD per patient.22 The collective evidence from our study suggests that an OAC alone strategy could be considered for these patients, and future studies comparing it with other strategies including APT alone and combination with APT in a prospective randomized setting are warranted.
Our study provides clinically relevant insights regarding the utilization of different antithrombotic regimens, and their association with long-term cardiovascular outcomes. However, several limitations should be considered. First, our study has limitations inherent to any observational study. However, the observational nature of our study provided us the opportunity to provide insights about real-world practice. Confounding by indication could be present, and patients receiving APT alone could present higher bleeding risk than patients who received OAC alone or in combination with APT. For this reason, the extrapolation of the results of our study in patients with concomitant coronary artery disease or percutaneous coronary intervention should be made with caution despite the statistical adjustments performed. We used inverse probability weighting to reduce imbalances in baseline characteristics across patients receiving different antithrombotic therapies, and the adjusted and unadjusted analyses presented consistent results. In addition, our study is based upon data from 2011–2015, and practice patterns with APT and OAC may be different now, given the rapid expansion of DOAC use and the completion of trials on APT with OAC use in PCI within the last year. However, our study remains the best representation of current practice patterns available for antiplatelet and antithrombotic strategies specifically for patients with AF undergoing TAVR Finally, we included patients with preexisting AF; as such, the results may not apply to patients with new-onset AF following TAVR.
Conclusions
In a large, real-world, contemporary cohort of patients with AF who undergo TAVR across the United States there is significant variability in the prescription of antithrombotic therapies, including underuse of OAC in over 40% of patients. The use of OAC+APT (vs. OAC alone or APT alone) did not lead to a reduced risk of stroke or all-cause mortality but was associated with increased risk of bleeding complications at 1-year compared with APT alone. Our findings are hypothesis generating with important clinical implications for this rapidly growing, highly vulnerable population and warrant validation in subsequent randomized clinical trials.
What is Known.
Atrial fibrillation (AF) is a common comorbid medical condition in patients undergoing transcatheter aortic valve replacement (TAVR)
Patients with AF undergoing TAVR have worse outcomes than those without AF
There is a lack of evidence for optimal antithrombotic therapy in patients with AF undergoing TAVR and current guidelines vary in their recommendations
What the Study Adds.
In a large, real-world, contemporary cohort of patients with AF undergoing TAVR in the U. S. there is significant variability in the prescription of antithrombotic therapies
There is significant underuse of oral anticoagulation (OAC) in patients with AF after undergoing TAVR
The use of OAC with antiplatelet therapy (APT) was not associated with a lower risk of stroke or mortality vs. OAC alone, but was associated with a greater risk of bleeding vs. APT alone
Acknowledgments
Sources of Funding: Dr. Gupta is supported by NIH training grant T32 HL007854.
Disclosures: Dr Sherwood reports personal fees from Medtronic outside the submitted work. Dr Gupta reports other from Edwards Lifesciences, other from Arnold Porter Law firm, other from Ben C. Martin law firm, and other from Heartbeat Health Inc outside the submitted work. Dr Vemulapalli reports grants from American College of Cardiology and grants from Society of Thoracic Surgeons during the conduct of the study; grants and personal fees from Boston Scientific and grants from Abbott outside the submitted work; and National Institutes of Health (R01 and SBIR) Janssen (advisory board), boston scientific (advisory board), HeartFlow (consulting), American College of Physicians (consulting). Dr Piccini reports grants from Abbott, grants from Bayer, grants from American Heart Association, grants from Philips, grants from Boston Scientific, and other from Medtronic outside the submitted work. Dr Harrison reports grants from Edwards Lifesciences, grants from Medtronic Inc, grants from Abbott/St Jude, and grants from Boston Scientific during the conduct of the study. Dr Vora reports personal fees from Medtronic during the conduct of the study. Dr Mack reports non-financial support from abbott, non-financial support from edwards lifesciences, and non-financial support from Medtronic during the conduct of the study. Dr Cohen reports grants and personal fees from Edwards Lifesciences, grants and personal fees from Medtronic, grants and personal fees from Boston Scientific, and grants and personal fees from Abbott outside the submitted work. Dr Kirtane reports Institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for speaking engagements and/or consulting. Personal: Consulting: Neurotronic; Travel Expenses/Meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr Peterson reports grants and personal fees from BMS, grants and personal fees from Janssen, grants from sanofi, grants and personal fees from pfizer, grants and personal fees from astraZeneca, and grants and personal fees from Amgen during the conduct of the study. All others have none.
Nonstandard Abbreviations and Acronyms
- TAVR
transcatheter aortic valve replacement
- TVT
transcatheter valve therapy
- STS
Society of Thoracic Surgeons
- ACC
American College of Cardiology
- CMS
Centers for Medicare and Medicaid Services
References:
- 1.Genereux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP and Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. Journal of the American College of Cardiology. 2012;59:2317–26. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, de Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, Friedman J, Berman D, Cheng W, Kashif M, Jelnin V, Kliger CA, Guo H, Pichard AD, Weissman NJ, Kapadia S, Manasse E, Bhatt DL, Leon MB and Sondergaard L. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. The New England journal of medicine. 2015;373:2015–24. [DOI] [PubMed] [Google Scholar]
- 3.Hansson NC, Grove EL, Andersen HR, Leipsic J, Mathiassen ON, Jensen JM, Jensen KT, Blanke P, Leetmaa T, Tang M, Krusell LR, Klaaborg KE, Christiansen EH, Terp K, Terkelsen CJ, Poulsen SH, Webb J, Botker HE and Norgaard BL. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. Journal of the American College of Cardiology. 2016;68:2059–2069. [DOI] [PubMed] [Google Scholar]
- 4.Tarantini G, Mojoli M, Windecker S, Wendler O, Lefevre T, Saia F, Walther T, Rubino P, Bartorelli AL, Napodano M, D’Onofrio A, Gerosa G, Iliceto S and Vahanian A. Prevalence and Impact of Atrial Fibrillation in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: An Analysis From the SOURCE XT Prospective Multicenter Registry. JACC Cardiovasc Interv. 2016;9:937–46. [DOI] [PubMed] [Google Scholar]
- 5.Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, Tousek P, van der Kley F, Buysschaert I, Schotborgh CE, Ferdinande B, van der Harst P, Roosen J, Peper J, Thielen FWF, Veenstra L, Chan Pin Yin D, Swaans MJ, Rensing B, van ‘t Hof AWJ, Timmers L, Kelder JC, Stella PR, Baan J and Ten Berg JM. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. The New England journal of medicine. 2020;382:1696–1707. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL and Group ESCSD. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 7.Asgar AW, Ouzounian M, Adams C, Afilalo J, Fremes S, Lauck S, Leipsic J, Piazza N, Rodes-Cabau J, Welsh R, Wijeysundera HC and Webb JG. 2019 Canadian Cardiovascular Society Position Statement for Transcatheter Aortic Valve Implantation. Can J Cardiol. 2019;35:1437–1448. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd and Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017;70:252–289. [DOI] [PubMed] [Google Scholar]
- 9.Wasfy JH, Kennedy KF, Chen JS, Ferris TG, Maddox TM and Yeh RW. Practice Variation in Triple Therapy for Patients With Both Atrial Fibrillation and Coronary Artery Disease: Insights From the ACC’s National Cardiovascular Data Registry. JACC Clin Electrophysiol. 2016;2:36–43. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood MW, Piccini JP, Holmes DN, Pieper KS, Steinberg BA, Fonarow GC, Allen LA, Naccarelli GV, Kowey PR, Gersh BJ, Mahaffey KW, Singer DE, Ansell JE, Freeman JV, Chan PS, Reiffel JA, Blanco R, Peterson ED and Rao SV. Outcomes of Cardiac Catheterization in Patients With Atrial Fibrillation on Anticoagulation in Contemporary in Practice: An Analysis of the ORBIT II Registry. Circulation Cardiovascular interventions. 2020;13:e008274. [DOI] [PubMed] [Google Scholar]
- 11.Ogilvie IM, Newton N, Welner SA, Cowell W and Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 12.Ailawadi G and Iung B. The TVT Registry: Collaboration Leading to Quality Control. Journal of the American College of Cardiology. 2017;69:1231–1233. [DOI] [PubMed] [Google Scholar]
- 13.Brennan JM, Edwards FH, Zhao Y, O’Brien S, Booth ME, Dokholyan RS, Douglas PS, Peterson ED and Team DEAR. Early anticoagulation of bioprosthetic aortic valves in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Journal of the American College of Cardiology. 2012;60:971–7. [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Jawad Altisent O, Durand E, Munoz-Garcia AJ, Nombela-Franco L, Cheema A, Kefer J, Gutierrez E, Benitez LM, Amat-Santos IJ, Serra V, Eltchaninoff H, Alnasser SM, Elizaga J, Dager A, Garcia Del Blanco B, Ortas-Nadal Mdel R, Marsal JR, Campelo-Parada F, Regueiro A, Del Trigo M, Dumont E, Puri R and Rodes-Cabau J. Warfarin and Antiplatelet Therapy Versus Warfarin Alone for Treating Patients With Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2016;9:1706–17. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DR Jr., Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D and Thomas JD. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. Journal of the American College of Cardiology. 2012;59:1200–54. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg BA, Kim S, Thomas L, Fonarow GC, Hylek E, Ansell J, Go AS, Chang P, Kowey P, Gersh BJ, Mahaffey KW, Singer DE, Piccini JP and Peterson ED. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, He W, Xian Y, Ciobanu AO, Kamath DY, Fox KA, Rao MP, Pokorney SD, Berwanger O, Tajer C, de Barros ESPGM, Roettig ML, Huo Y, Granger CB and investigators I-A. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017;390:1737–1746. [DOI] [PubMed] [Google Scholar]
- 18.Hess PL, Mirro MJ, Diener HC, Eikelboom JW, Al-Khatib SM, Hylek EM, Bosworth HB, Gersh BJ, Singer DE, Flaker G, Mega JL, Peterson ED, Rumsfeld JS, Steinberg BA, Kakkar AK, Califf RM and Granger CB. Addressing barriers to optimal oral anticoagulation use and persistence among patients with atrial fibrillation: Proceedings, Washington, DC, December 3–4, 2012. American heart journal. 2014;168:239–247.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosmidou I, Liu Y, Alu MC, Liu M, Madhavan M, Chakravarty T, Makkar R, Thourani VH, Biviano A, Kodali S and Leon MB. Antithrombotic Therapy and Cardiovascular Outcomes After Transcatheter Aortic Valve Replacement in Patients With Atrial Fibrillation. JACC Cardiovasc Interv. 2019;12:1580–1589. [DOI] [PubMed] [Google Scholar]
- 20.Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sørensen R, Køber L, Torp-Pedersen C and Hansen ML. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–85. [DOI] [PubMed] [Google Scholar]
- 21.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH and Investigators A. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. The New England journal of medicine. 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SV, Lei Y, Reynolds MR, Magnuson EA, Suri RM, Tuzcu EM, Petersen JL 2nd, Douglas PS, Svensson LG, Gada H, Thourani VH, Kodali SK, Mack MJ, Leon MB, Cohen DJ and Investigators P. Costs of periprocedural complications in patients treated with transcatheter aortic valve replacement: results from the Placement of Aortic Transcatheter Valve trial. Circulation Cardiovascular interventions. 2014;7:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]