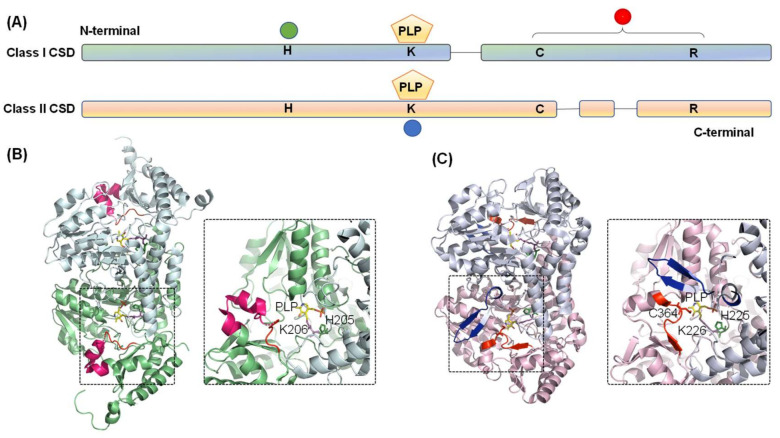
Figure 3.
Diagram representing the domain architecture of CSD from Classes I and II. (A) The green circle indicates conserved histidine residue involved in acid-base catalysis and interaction with PLP-L-cysteine. Conserved cysteine and arginine residues are in the catalytic loop region, as indicated by the red circle. PLP is pyridoxal-5′-phosphate is covalently bound to conserved lysine residue (blue circle). Comparison of the three-dimensional structures of the (B) Class I (E. coli IscS, PDB ID- 3LVM) and (C) Class II (E. coli SufS, PDB ID- 6UY5) CSD enzymes highlights the differences between the two classes. Conserved histidine, lysine, and catalytic cysteine residues are shown as stick representation in forest green, magenta, and red. The PLP moiety is shown in yellow. Identifiers I–III are highlighted in red, blue, and hot pink, respectively.