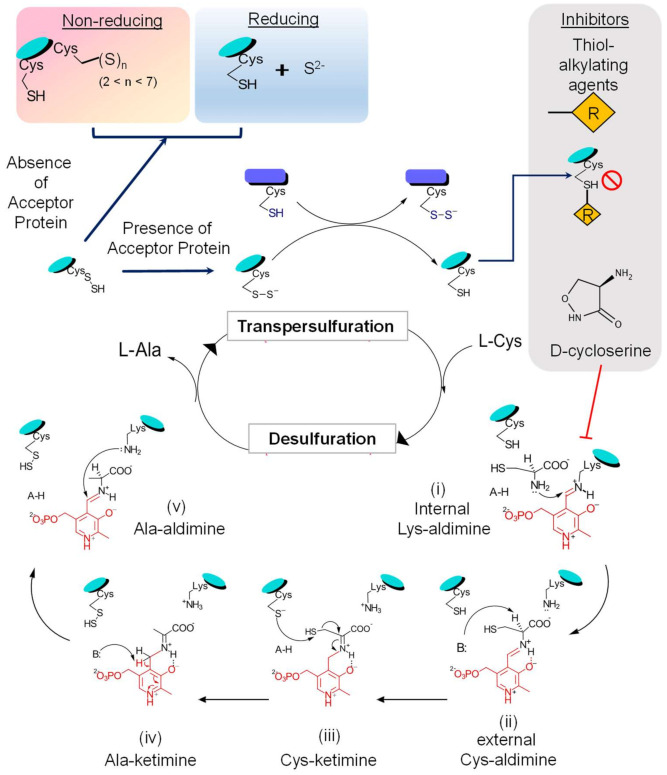
Figure 4.
Schematic representation of the CSD reaction mechanism. The enzymatic activity of CSD can be divided into two phases: firstly, desulfuration of substrate L-cysteine and formation of the enzyme-bound persulfide intermediate, followed by the transfer of persulfide to its acceptor proteins, transpersulfuration. During the first phase of the reaction, the covalently bound cofactor-PLP acts as an electron sink and aids in the nucleophilic attack and the concomitant S-abstraction. This reaction proceeds via the formation of several reactive intermediate steps. The outcome of transpersulfuration can depend on the adjacent redox environment and the availability of S-acceptor proteins. On interaction with S-acceptor proteins, there is a transfer of the CSD-bound persulfide to the reactive thiol of an active site cysteine residue in the acceptor protein. However, in the absence of acceptors, the persulfide can get released as S2−/H2S under reducing conditions or form polysulfide species Sn (2 < n < 7) on an adjacent free thiol under non-reducing conditions. Furthermore, the activity of CSD can be inhibited by thiol-alkylating agents (such as N-ethylmaleimide, iodoacetamide) by irreversibly modifying the thiol group of the active cysteine residue. CSDs, being PLP-dependent enzymes, are inhibited by molecules that covalently interact with the PLP-moiety forming irreversible protein-inhibitor complexes (for example, D- or L-cycloserine).