FIGURE 1.
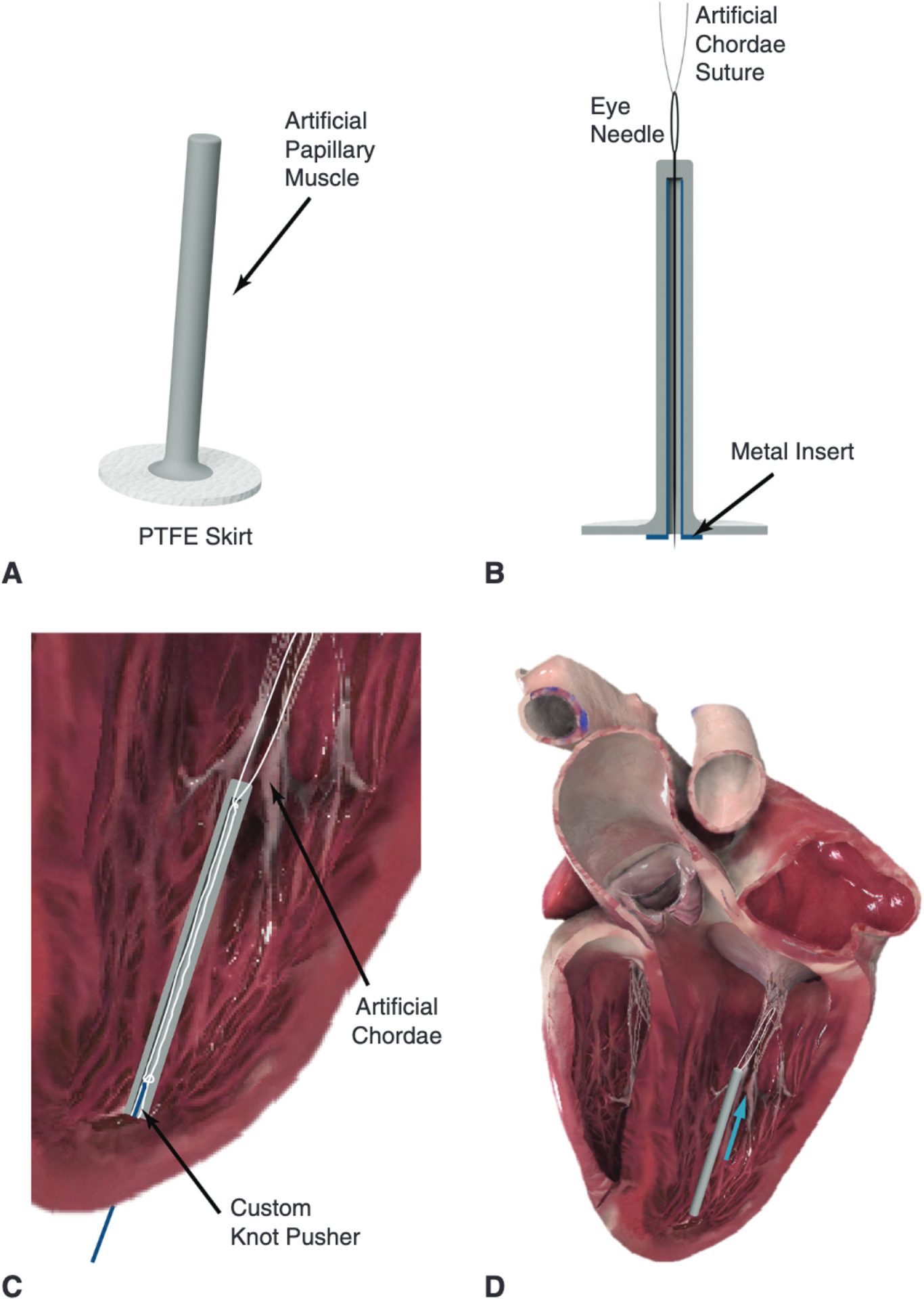
A, Labeled rendering of the artificial papillary muscle device to be used in conjunction with a minimally invasive transapical artificial chordae implantation device. Multiple pairs of chords can be anchored to this artificial papillary muscle. B, An eye needle is used to thread each artificial chordae suture end through the head of the artificial papillary muscle and down through its inner core; a metal insert can be used to facilitate this threading process. C, A cross-section of the artificial papillary muscle showing the suture length adjustment. The length for each pair of sutures would be determined under echo guidance and could be set with a low-profile knot pusher or any crimping device. D, The final implanted artificial papillary muscle serves to shorten the artificial chordae and attenuate high rate of change of force; blue arrow represents force from the suture. PTFE, Polytetrafluoroethylene. Heart images courtesy of Complete Anatomy.
