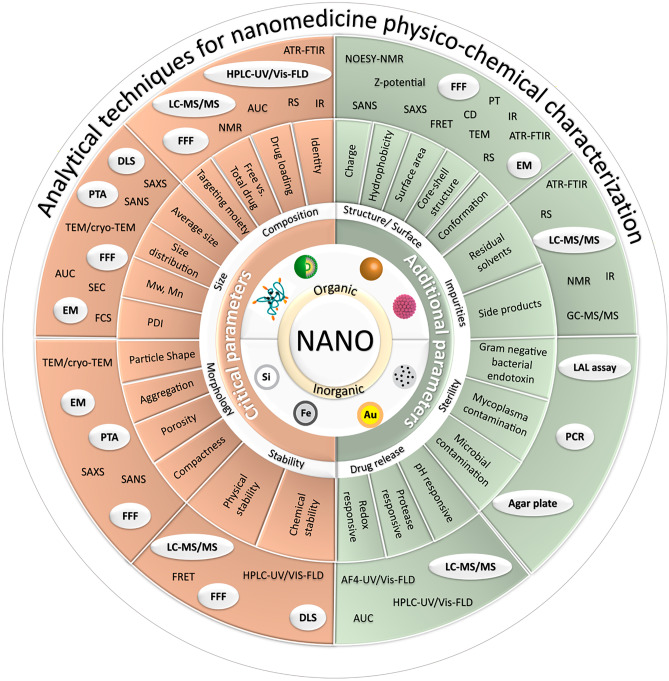
Fig. 3.
Critical and additional parameters of nanomedicines and corresponding analytical techniques for their characterization (NCL approved techniques are highlighted). LC–MS/MS liquid chromatography–mass spectrometry, FFF field flow fractionation, NMR nuclear magnetic resonance, AUC analytical ultracentrifugation, RS remote sensing, IR infrared, HPLC–UV high-performance liquid chromatography-ultraviolet, Vis-FLD visible-fluorescence detector, ATR-FTIR attenuated total reflection—Fourier transform infrared spectroscopy, NOESY-NMR nuclear Overhauser effect spectroscopy-nuclear magnetic resonance, SANS small angle neutron scattering, SAXS small angle x-ray scattering, FRET fluorescence resonance energy transfer, CD circular dichroism, PT potentiometry, TEM transmission electron microscopy, EM electron microscopy, GC–MS/MS gas chromatography–mass spectrometry, LAL assay limulus amebocyte lysate assay, PCR polymerase chain reaction, AF4-UV asymmetric field flow fractionation-ultraviolet, DLS dynamic light scattering, Cryo-TEM cryogenic transmission electron microscopy, FCS fluorescence correlation spectroscopy, SEC size-exclusion chromatography, PTA particle tracking analysis, Si silicon, Fe iron, Au gold