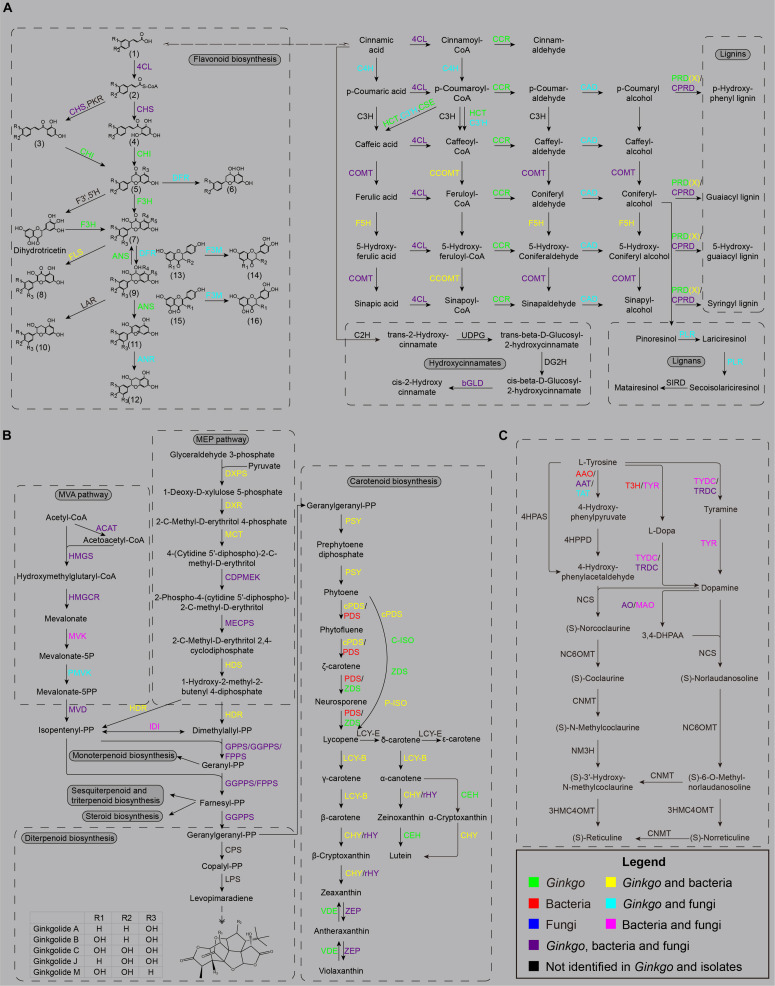
FIGURE 4.
The main secondary metabolism pathways. (A) The biosynthesis sketch of flavonoid and non-flavonoid polyphenolics in the Ginkgo root community. The R labels represent a hydrogen atom, a hydroxyl, or a methoxy in all chemical structures. (1) trans-cinnamic acid; p-coumaric acid; caffeic acid; ferulic acid; (2) cinnamoyl-CoA; p-coumaroyl-CoA; caffeoyl-CoA; (3) isoliquiritigenin; butein; (4) pinocembrin chalcone; naringenin chalcone; eriodictyol chalcone; (5) pinocembrin; liquiritigenin; butin; naringenin; eriodictyol; (6) apiforol; luteoforol; (7) pinobanksin; garbanzol; fustin; dihydrokaempferol; dihydroquercetin; dihydromyricetin; (8) galangin; kaempferol; quercetin; myricetin; (9) 5-deoxyleucopelargonidin; 5-deoxyleucocyanidin; cis-3, 4-leucopelargonidin; leucocyanidin; leucodelphinidin; (10) afzelechin; (+)-catechin; (+)-gallocatechin; (11) pelargonidin; cyanidin; delphinidin; (12) (–)-epiafzelechin; (–)-epicatechin; (–)-epigallocatechin; (13) liquiritigenin; garbanzol; naringenin; dihydrokaempferol; (14) butin; fustin; eriodictyol; dihydroquercetin; (15) apigenin; kaempferol; (16) luteolin; quercetin. (B) The biosynthesis sketch of terpenoid backbone and their derivatives in the Ginkgo root community. (C) The biosynthesis sketch of isoquinoline alkaloid backbone in the Ginkgo root community.