Figure 3.
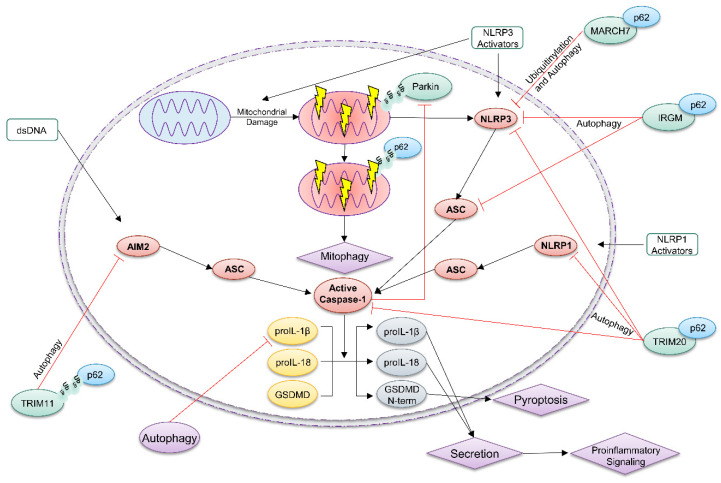
p62-dependent autophagy antagonizes inflammasomes. The inflammasome sensors NLRP3, NLRP1 and AIM2 sense certain stressors, which induce their activation. This causes oligomerization of the adaptor protein ASC (speck formation) and in turn activation of the protease caspase-1 [81]. Subsequently, caspase-1 activates the proinflammatory cytokine proIL-1β and -18 by proteolytic processing. Release of mature IL-1β and -18 induces an inflammatory response, which is dependent on caspase-1-dependent cleavage and activation of GSDMD. p62 is required for mitophagy of damaged mitochondria that induce NLRP3 activation [90]. Moreover, MARCH7 ubiquitinates NLRP3, inducing its p62-dependent selective autophagic degradation [91]. Together with p62, IRGM regulates degradation of NLRP3 and ASC via autophagy [92]. The E3 ubiquitin ligase TRIM20 ubiquitinates NLRP3, NLRP1 and caspase-1, thereby initiating their p62-dependent autophagic clearance [93]. Ubiquitinated TRIM11 binds with p62 to AIM2 causing its degradation by autophagy [94].