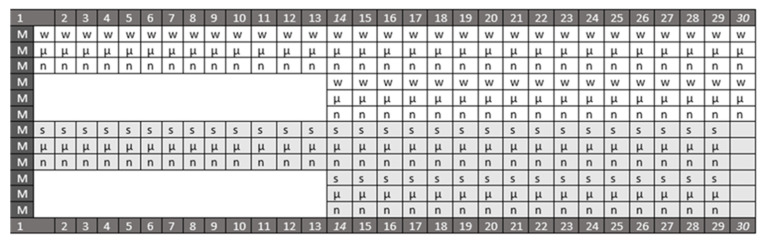
Figure 1.
Shematic presentation of the given protocol. Dark gray rows (white letters) indicate days (1–30; 14 and 30 indicate days of sacrifice, or later initiation of the therapy) after monocrotaline (M) (80 mg/kg subcutaneously on day 1, as described before [17]). Stable gastric pentadecapeptide BPC 157 medication (10 μg/kg (μ) or 10 ng/kg (n)) (as reviewed in [1,2,3,4,5,6,7,8,9,10,11,12,13,14]) was given intraperitoneally (light gray rows) or per-orally in drinking water (white rows); controls received an equal volume of saline (5 mL/kg intraperitoneally) (s) or drinking water (w).