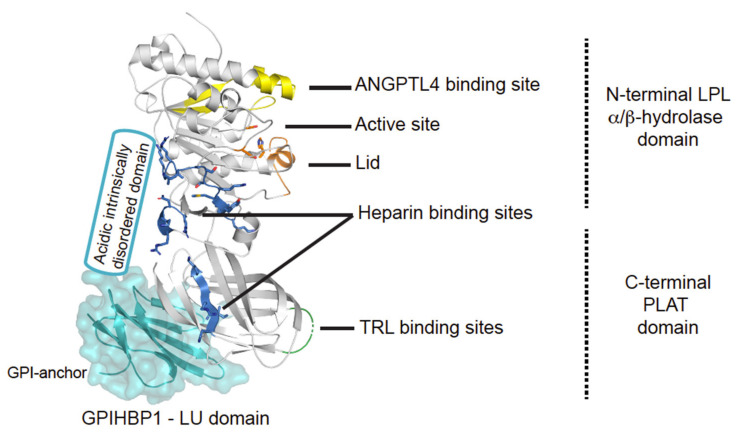
Figure 2.
Structural elements in LPL. Cartoon representation of the human LPL•GPIHBP1 complex and with the molecular surface of the LU domain (Ly6/uPAR protein domain family) of GPIHBP1 shown as a transparent light blue envelope (generated with The PyMol Molecular Graphic System (Version 2.0 Schrödinger, LLC) using coordinates from the LPL•GPIHBP1 crystal structure; Protein Data Bank ID code 6E7K). LPL elements implicated in ANGPTL4 binding are highlighted in yellow [17]. GPIHBP1 binds to the C-terminal PLAT domain of LPL, and the location of GPIHBP1’s membrane-tethering site (GPI anchor) is indicated [18]. GPIHBP1’s acidic intrinsically disordered domain stabilizes LPL’s α/β-hydrolase domain and is assumed to interact with a large basic patch on the surface of LPL [19,20]. The active site containing the catalytic triad (Ser134, Asp158, and His243) is represented with orange residues [21] and the orange helix is the lid covering the active site responsible for substrate specificity [22]. The triglyceride-rich lipoprotein (TRL) binding site is indicated in green and residues important for heparin binding is shown in blue [23,24,25].