Figure 3.
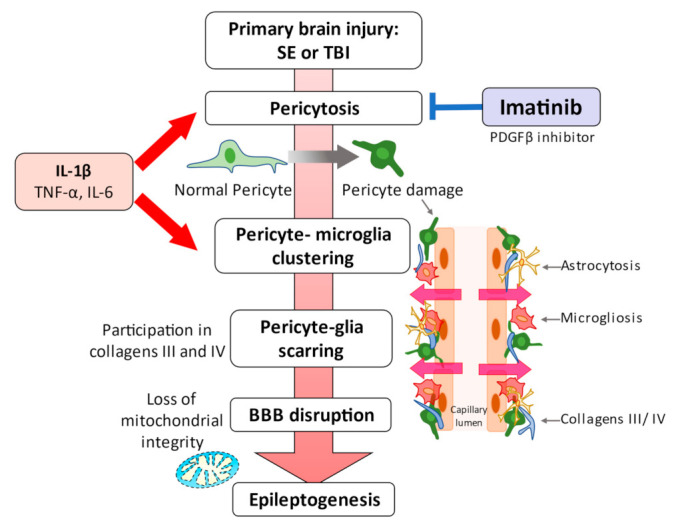
Schematic representation of the events linking pericytes to epilepsy. Status epilepticus leads to redistribution and remodeling of cerebrovascular pericytes, potentially contributing to blood–brain barrier permeability [2,28,29]. A significant clustering of microglia/macrophages around pericytes occurs one week after the attack, although pericyte proliferation is significantly increased as early as 72 h [29]. These series of pericyte-related modifications are promoted by proinflammatory cytokines, including IL-1β, TNFα, and IL-6. Alterations caused by IL-1β, which is one of the cytokines most deeply involved in the pathogenesis of epilepsy, were most pronounced. These pericyte-associated modifications and pericyte-microglia clustering may be facilitated by IL-1β [29], and pericyte-glial scarring with collagens III and IV process leaky capillaries during seizure progression [30]. Recurrent seizures can lead to pericytic injury with neurovascular decoupling and BBB dysfunction at the arterial and capillary levels. Moreover, capillary vasoconstriction is accompanied by a loss of mitochondrial integrity in pericytes [81]. In vitro and in vivo studies have highlighted the potential of pericytes as a therapeutic target for seizure disorders [28,30,32].
