Abstract
Studies investigating the effects of tocotrienols on inflammation and oxidative stress have yielded inconsistent results. This systematic review and meta-analysis aimed to evaluate the effects of tocotrienols supplementation on inflammatory and oxidative stress biomarkers. We searched PubMed, Scopus, and Cochrane Central Register of Controlled Trials from inception until 13 July 2020 to identify randomized controlled trials supplementing tocotrienols and reporting circulating inflammatory or oxidative stress outcomes. Weighted mean difference (WMD) and corresponding 95% confidence interval (CI) were determined by pooling eligible studies. Nineteen studies were included for qualitative analysis, and 13 studies were included for the meta-analyses. A significant reduction in C-reactive protein levels (WMD: −0.52 mg/L, 95% CI: −0.73, −0.32, p < 0.001) following tocotrienols supplementation was observed, but this finding was attributed to a single study using δ-tocotrienols, not mixed tocotrienols. There were no effects on interleukin-6 (WMD: 0.03 pg/mL, 95% CI: −1.51, 1.58, p = 0.966), tumor necrosis factor-alpha (WMD: −0.28 pg/mL, 95% CI: −1.24, 0.68, p = 0.571), and malondialdehyde (WMD: −0.42 μmol/L, 95% CI: −1.05, 0.21, p = 0.189). A subgroup analysis suggested that tocotrienols at 400 mg/day might reduce malondialdehyde levels (WMD: −0.90 μmol/L, 95% CI: −1.20, −0.59, p < 0.001). Future well-designed studies are warranted to confirm the effects of tocotrienols on inflammatory and oxidative stress biomarkers, particularly on different types and dosages of supplementation. PROSPERO registration number: CRD42020198241.
Introduction
Inflammation is an essential immune response for immunosurveillance and host defense. While acute inflammation is a beneficial and self-limiting process to eliminate toxic agents or promote the repair of damaged tissue, chronic inflammation is a pathological state associated with a homeostatic imbalance of physiological systems not directly triggered by infection or injury [1,2]. On the other hand, oxidative stress is a phenomenon that denotes an imbalance between the production and accumulation of free radicals or oxidants and the antioxidant system to counteract these reactive products [3]. Chronic inflammation and oxidative stress are two pathophysiological conditions that coexist because they can induce each other mutually [4]. Chronic inflammation and oxidative stress disrupt normal cellular physiology. They have been implicated in the development and progression of various metabolic or chronic diseases such as metabolic syndrome and diabetes, cardiovascular disease, cancers, neurodegenerative disorders, chronic kidney disease, liver disease, and rheumatoid arthritis [3,5,6].
Vitamin E, also known as α-tocopherol, is a lipid-soluble vitamin with potent antioxidant and anti-inflammatory properties. Tocopherols possess a saturated wide chain while tocotrienols differ from tocopherols by possessing an isoprenoid side chain with three double bonds. [7,8]. Tocotrienols are further divided into four distinct isomers: alpha (α), beta (β), gamma (γ), and delta (δ). Till now, most research has focused primarily onα-tocopherol. However, there is a growing interest in exploring the potential role of tocotrienols in preventing and treating chronic diseases [9]. Tocotrienols are reported to possess more superior antioxidant properties than α-tocopherol due to the presence of the unsaturated side chain, which allows more efficient incorporation into tissues with saturated fatty layers such as the brain and liver [10]. Beyond the antioxidant capacity, tocotrienols are also shown to modulate inflammatory responses via the regulation of gene expression of pro-inflammatory cytokines [11]. Tocotrienols are naturally found in edible oils such as palm oil, annatto oil, rice bran oil, and coconut oil [10]. A recent meta-analysis of 26 clinical trials showed that vitamin E supplementation significantly reduced serum C-reactive protein (CRP) levels [12]. However, this meta-analysis included studies investigating both tocopherols and tocotrienols, and subgroup analysis specific to tocotrienols was not performed. Thus, the anti-inflammatory effect of tocotrienols could not be confirmed. In addition, there has been no systematic review examining the clinical evidence on the antioxidant effects of tocotrienols. Therefore, the present systematic review aimed to evaluate the effects of tocotrienols supplementation compared to placebo on inflammatory and oxidative stress biomarkers.
Methods
Study protocol
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [13], and the protocol for this systematic review was registered on PROSPERO (CRD42020198241).
Search strategy
We performed a comprehensive search in PubMed, Scopus, and Cochrane Central Register of Controlled Trials on 13 July 2020 to identify relevant studies. Medical Subject Headings terms and free-text terms for randomized controlled trials and tocotrienols were used as the search strategy (S1 Table). We did not apply keywords specific to biomarkers of inflammation and oxidative stress in the search strategy because we intended to search for all available randomized controlled trials investigating the effects of tocotrienols supplementation. We also manually searched for potential articles by checking the reference lists of relevant original articles, narrative reviews, systematic reviews, and meta-analyses from inception to July 2020.
Study inclusion and exclusion criteria
Randomized controlled trials (parallel or crossover) were eligible for inclusion in the current systematic review if they (i) compared tocotrienols supplementation versus placebo, (ii) had an intervention for at least two weeks, and (iii) reported at least one blood biomarker of inflammation or oxidative stress before and after administration of tocotrienols. We excluded (i) in vitro or animal studies, (ii) non-randomized or single-arm studies, (iii) in vivo acute or postprandial studies, (iv) studies with combinations of tocotrienols and other dietary components, (v) studies reporting outcome measures such as urinary markers, gene expression, or in vitro stimulated inflammatory response, (vi) unpublished articles, abstracts, conference proceedings, or letters, and (vii) non-English publications. We screened for duplicate publications (articles based on the same dataset) through the trial registration number, list of authors, subjects’ baseline characteristics, and funder. We only included the duplicate publication with the larger subject number or longer duration of intervention.
Study selection and data extraction
Citations from the initial search results of each database were exported to EndNote (version X7.5.3, Clarivate Analytics, Philadelphia, PA, USA), and duplicates were removed. The titles and abstracts were screened and reviewed by two authors (B-H.K. and H-C.T.). Then, full texts of potential studies were retrieved and independently reviewed in detail for inclusion based on the pre-determined criteria. Discrepancies between two authors were resolved by discussion, and a third author (A.H.A.G.) was referred if consensus could not be reached.
One author (H-C.T.) extracted the data from the included studies into a piloted sheet, and another author (B-H.K) crosschecked the extracted data. The following data were extracted: study characteristics (country, sample size, and design), subjects’ characteristics (age, sex, and population), intervention (type, dosage, duration, and placebo), biomarkers of inflammation and oxidative stress (baseline, post-intervention, and/or changes between baseline and post-intervention), and study funders. We only included data of the longest follow-up for studies with multiple time points of follow-up.
Three studies had incomplete information to derive the mean difference, and four studies presented the data in median (interquartile range) or figures. Therefore, we contacted the authors of these seven studies for data requests. However, the authors of a study published in 1996 were unreachable. Thus the study was excluded from the quantitative analysis. Out of six authors contacted, five authors had provided us with the necessary data, while one author could not respond to our request. Therefore, we used Plot Digitizer (http://plotdigitizer.sourceforge.net) to extract the data that was presented in a bar chart. Data extracted using this software was 74% agreement with the original data [14].
Assessment of risk of bias
The risk of bias of all included studies was assessed independently by two authors (B-H.K. and H-C.T.) using The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials [15], which consisted of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Statistical analyses
Meta-analyses were performed for biomarkers of inflammation and oxidative stress reported by at least three studies, while a descriptive summary of findings was presented for other biomarkers. The weighted mean differences (WMD) with corresponding 95% confidence intervals (CI) were determined by pooling eligible studies for meta-analyses. Heterogeneity among the studies was assessed using the chi-squared and I2 statistics, whereby a p-value < 0.1 and I2 above 50% indicates having significant heterogeneity, and a random effect model was used. We also performed subgroup analysis to explore the potential source of heterogeneity among studies, according to sample size (≥ 60 or less), duration of the intervention (≥ 6 months or less), and tocotrienols dosage (≥ 400 mg/day or less). A sensitivity analysis was performed by sequentially omitting one study at a time to verify that any single study did not influence the overall result. Publication bias was assessed by using Begg’s and Egger’s tests, and visually inspecting the symmetry of the funnel plot. STATA software (version 16.0, StataCorp, College Station, TX, USA) was used for the analysis.
Results
The flow of study selection
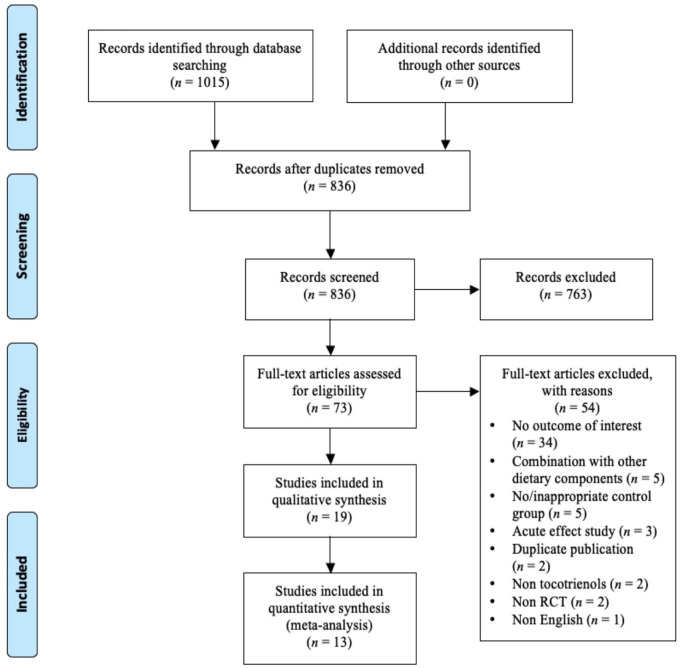
The flowchart of study selection is presented in Fig 1. From the literature search, 836 unique citations were identified from three databases. After screening the title and abstract, 73 studies were retrieved for full-text review. From this, 54 studies were excluded, and the reasons for exclusion are presented in the S2 Table. Out of the 19 studies [16–34] included in the present systematic review, there were four studies [16,17,22,23] involving subjects from two similar datasets, but these studies were included because each study reported unique biomarkers. For other duplicate publications, Pervez et al. [30] was selected due to a larger sample size, while Kooyenga et al. [25] was selected due to a longer follow-up duration. Thirteen studies [17–24,26,28,30,32,34] reporting CRP or high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), or malondialdehyde (MDA) were included for meta-analyses.
Fig 1. Flow diagram of study selection.
Study characteristics
The characteristics of 19 studies included in the systematic review are presented in Table 1. There were 17 parallel studies [16–19,21–26,28–34] and two crossover studies [20,27]. Majority of these studies were carried out in Malaysia (n = 12) [16–18,20,21,24,26–28,31,33,34], followed by United States of America (n = 3) [19,25,29], Iran (n = 2) [22,23], Pakistan (n = 1) [30] and Australia (n = 1) [32]. The sample size ranged from 31 to 121 subjects, with only one study [21] having sample size above 100 subjects. The mean age of subjects ranged from 23.3 to 66.6 years old. The dose of tocotrienols supplement ranged from 80 to 600 mg/day and the details are presented in S3 Table. The intervention duration ranged from 0.5 month to 24 months, and only three studies provided tocotrienols supplements for at least 12 months [21,25,26] while the remaining studies provided intervention for 6 months or less. Most studies (n = 7) recruited patients with type 2 diabetes mellitus [22,23,27,28,32–34], followed by healthy individuals (n = 4) [16–18,31], metabolic syndrome (n = 2) [20,24], hypercholesterolemia (n = 1) [29], non-alcoholic fatty liver disease (n = 1) [30], hypercholesterolemia and non-alcoholic fatty liver disease (n = 1) [26], white matter lesion (n = 1) [21], end-stage kidney disease on hemodialysis (n = 1) [19], and coronary artery disease (n = 1) [25]. For inflammatory markers, eight studies reported CRP or hsCRP [19–22,24,26,30,32], four studies reported IL-6 [19,24,30,32], and three studies reported TNF-α [24,30,32]. For oxidative stress markers, seven studies reported MDA [17,18,22,27,28,30,34], two studies each reported advanced glycation end-products (AGEs) [18,33], protein carbonyl (PC) [17,18], and thiobarbituric acid reactive substances (TBARS) [19,25], respectively, and one study each reported low-density lipoprotein (LDL) oxidation status [29] and lipid oxidation products (LOPs) [25], respectively. For antioxidant enzyme activities and response, three studies reported plasma antioxidant response [19,22,31], two studies [16,18] reported erythrocyte superoxide dismutase (SOD) [16,18], catalase (CAT), and glutathione peroxidase (GPx), and one study [16] reported erythrocyte glutathione (GSH). Majority of the studies (n = 13) were funded by the Malaysian government or agencies [16–21,24,26–28,31–34], two studies were funded by an Iranian university [22,23], one study each was funded by a German company [29] and government of Pakistan [30], respectively, one study did not provide information on the study funder [25].
Table 1. Characteristics of studies and subjects included in this systematic review.
| No. | Author, year | Country | n | Sex (M/F) | Mean Age (yr) | Population | Design | Duration (month) | T3 type (dosage)* | Placebo | Biomarkers | Funder |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Azman, 2018 [16], Goon 2017 [17] | Malaysia | 47 | 17/30 | 52.8 | Healthy | Parallel | 6 | TRF (150 mg/d) | Olive oil | SOD, CAT, GPx, MDA, PC, GSH | UKM |
| 2. | Chin, 2011 [18] | Malaysia | 62 | 0/62 | ≥ 35 | Healthy | Parallel | 6 | TRF (160 mg/d) | Palm oil | SOD, CAT, GPx, PC, AGE, MDA | Malaysian government & UKM |
| 3. | Daud, 2013 [19] | USA | 81 | 43/38 | 58.5 | HD | Parallel | 4 | TRF (180 mg/d) | Wheat germ oil | CRP, IL-6, TAP, TBARS | MPOC |
| 4. | Gan, 2017 [20] | Malaysia | 31 | 15/16 | 37.9 | Metabolic syndrome | Cross-over | 0.5 | PTT (400 mg/d) | Palm olein | hsCRP | MBOB |
| 5. | Gopalan, 2014 [21] | Malaysia | 121 | 48/73 | 52.0 | Subjects with WML | Parallel | 24 | Mixed T3 (400 mg/d) | Palm olein | hsCRP | MPOB |
| 6. | Haghighat, 2013 [22], Vafa, 2015 [23] | Iran | 45 | 22/33 | 55.6 | T2DM | Parallel | 2 | T3-enriched canola oil (200 mg/d) | Canola oil | hsCRP, MDA, TAC | Tehran University of Medical Sciences |
| 7. | Heng, 2015 [24] | Malaysia | 57 | 20/37 | 41.2 | Metabolic syndrome | Parallel | 4 | Mixed T3 (400 mg/d) | Soy bean oil | CRP, IL-6, TNF-α | Malaysian MOHE |
| 8. | Kooyenga, 1997 [25] | USA | 50 | 23/27 | 66.6 | CAD | Parallel | 24 | α- and γ-T3 (160–240 mg/d) | Palm superolein | LOPs, TBARS | N/A |
| 9. | Magosso, 2013 [26] | Malaysia | 87 | 34/53 | 51.0 | Hypercho-lesterolemic & NAFLD | Parallel | 12 | Mixed T3 (400 mg/d) | Placebo† | hsCRP | MPOB |
| 10. | Nazaimoon, 1996 [27] | Malaysia | 32 | 9/23 | 41.3 | T2DM | Cross-over | 6 | T3 (288 mg/d) | Palm olein | MDA | MPOB |
| 11. | Ng, 2020 [28] | Malaysia | 80 | 52/28 | 63.5 | T2DM | Parallel | 2 | T3 (400 mg/d) | Palm oil | MDA | Malaysian MOHE & MUM |
| 12. | O’Byrne, 2000 [29] | USA | 51 | 22/29 | 40.8 | Hypercho-lesterolemic | Parallel | 2 | α- or γ- or δ- tocotrienyl acetate (250 mg/d) | MCT oil | LDL oxidation status | BASF (Germany) & NIH |
| 13. | Pervez, 2020 [30] | Pakistan | 71 | 34/37 | 44.4 | NAFLD | Parallel | 6 | δ-T3 (600 mg/d) | Sucrose | hsCRP, IL-6, TNF-α, MDA | Government of Pakistan |
| 14. | Rasool, 2006 [31] | Malaysia | 36 | 36/0 | 23.3 | Healthy | Parallel | 2 | TRE (80–320 mg/d) | N/A† | TAS | Malaysian MOSTI |
| 15. | Stonehouse, 2016 [32] | Australia | 57 | 36/21 | 60.8 | T2DM, IFG or elevated WC | Parallel | 2 | TRF (420 mg/d) | Palm olein | hsCRP, IL-6, TNF-α | MPOB |
| 16. | Tan, 2018 [33] | Malaysia | 45 | 31/14 | 61.6 | T2DM | Parallel | 2 | TRE (400 mg/d) | N/A† | AGE | MUM |
| 17. | Tan, 2019 [34] | Malaysia | 54 | 35/19 | 61.3 | T2DM | Parallel | 3 | TRE (400 mg/d) | N/A† | MDA | Malaysian MOHE & MUM |
*Detailed composition of the supplements is presented in S3 Table
† The composition of placebo was not stated.
Abbreviation: AGE, advanced glycosylation end-product, CAD, carotid artery disease, CAT, catalase, CRP, C-reactive protein, F, female, GPx, glutathione peroxidase, GSH, gluthathione, HD, hemodialysis, hsCRP, high sensitivity C-reactive protein, IFG, impaired fasting glucose, IL-6, interleukin-6, LDL, low-density lipoprotein, LOPs, lipid peroxidation products, M, male, MCT, medium chain triglyceride, MDA, malondialdehyde, MOSTI, Ministry of Science, Technology & Innovation Malaysia, MPOB, Malaysian Palm Oil Board, MPOC, Malaysian Palm Oil Council, MUM, Monash University Malaysia, N/A, not available, NAFLD, non-alcoholic fatty liver disease, NIH, National Institutes of Health, PC, protein carbonyl, PTT, palm based tocotrienols and tocopherol, SOD, superoxide dismutase, T2DM, type 2 diabetes mellitus, T3, tocotrienols, TAC, total antioxidant capacity, TAP, total antioxidant power, TAS, total antioxidant status, TBARS, thiobarbituric acid reactive substances, TNF-α, tumor necrosis factor-alpha, TRE, tocotrienol-rich vitamin E, TRF, tocotrienol-rich fraction, UKM, Universiti Kebangsaan Malaysia, USA, United States of America, WC, waist circumference, WML, white matter lesions.
Effects of tocotrienols supplementation on markers of inflammation
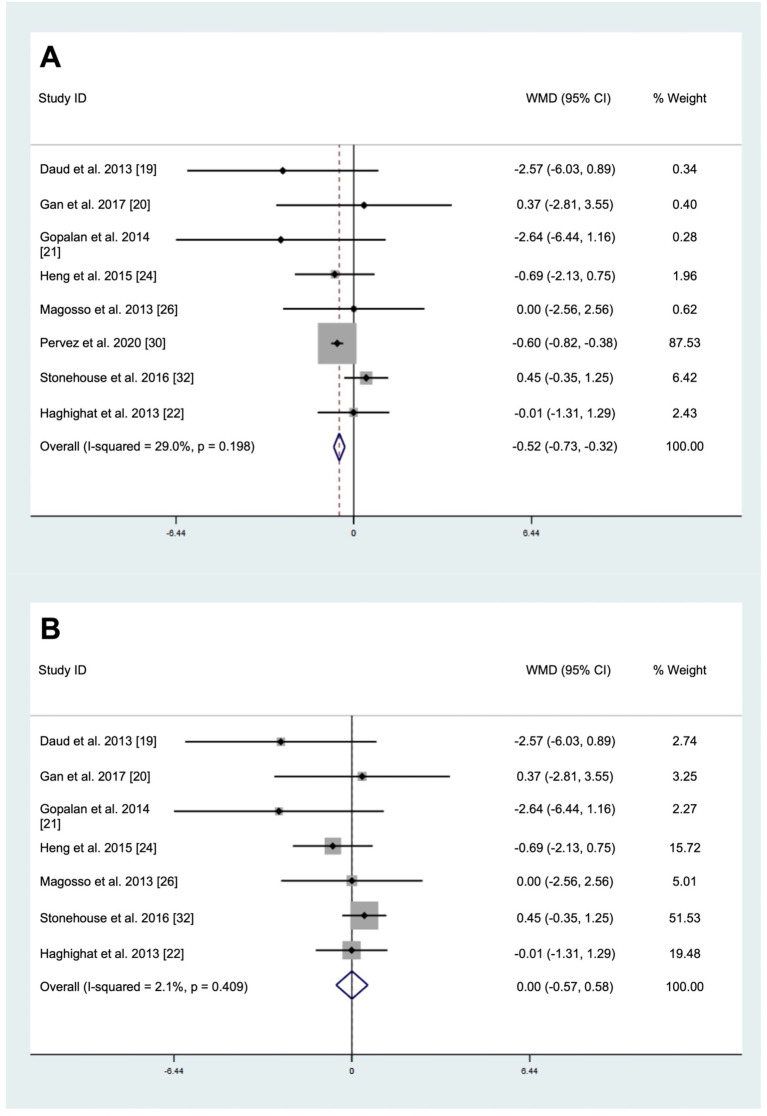
The pooled result of eight studies involving a total of 547 subjects (intervention = 275, placebo = 273) demonstrated a significant decrease in CRP levels after tocotrienols supplementation (WMD: -0.52 mg/L, 95% CI: -0.73, -0.32, p < 0.001, Fig 2) using the fixed-effect model as no significant heterogeneity was observed (I2 test = 29.0%, p = 0.196). Visual inspection of the funnel plot (S1 Fig), Egger’s test (p = 0.743), and Begg’s test (p = 0.458) indicated no publication bias. However, the sensitivity analysis (S2 Fig) showed that the omission of Pervez et al. [30] changed the results, indicating that the pooled result was primarily influenced by Pervez et al. [30], which accounted for 87.53% of the weightage. A separate analysis with exclusion of Pervez et al. [30] showed that supplementations of mixed tocotrienols did not affect CRP levels (WMD: 0 mg/L, 95% CI: -0.57, 0.58, p = 0.992, Fig 2) using the fixed-effect model as no significant heterogeneity was observed (I2 test = 2.1%, p = 0.409). Subgroup analyses showed that significant reductions in CRP levels were observed in study with sample size for at least 60 subjects (WMD: -0.61 mg/L, 95% CI: -0.82, -0.40, p < 0.001) and study for at least 6 months (WMD: -0.60 mg/L, 95% CI: -0.82, -0.39, p < 0.001) (Table 2).
Fig 2.
Forrest plot on the effect of tocotrienols supplementation on C-reactive protein levels for (A) analysis with all studies (B) analysis excluding Pervez et al. [30].
Table 2. Subgroup analyses of tocotrienols on C-reactive protein level.
| n | WMD (95% CI) | p within group | p heterogeneity | I2 (%) | |
|---|---|---|---|---|---|
| Study sample size | |||||
| ≥ 60 | 4 | -0.61 (-0.82, -0.40) | < 0.001 | 0.465 | 0 |
| < 60 | 4 | 0.15 (-0.46, 0.75) | 0.630 | 0.589 | 0 |
| Study duration | |||||
| ≥ 6 months | 3 | -0.60 (-0.82, -0.39) | < 0.001 | 0.517 | 0 |
| < 6 months | 5 | 0.07 (-0.53, 0.66) | 0.822 | 0.377 | 5.3 |
Abbreviation: CI, confidence interval, WMD, weighted mean difference.
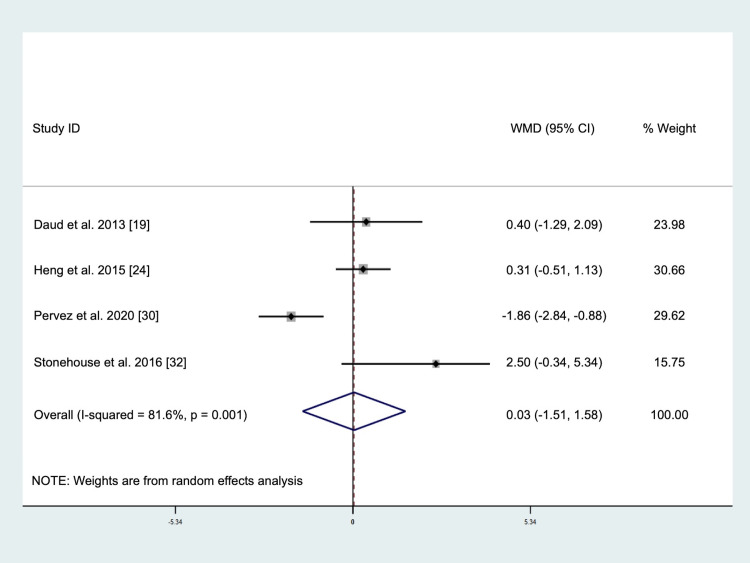
The pooled result of four studies involving a total of 261 subjects (intervention = 132, placebo = 129) demonstrated no significant changes in IL-6 levels after tocotrienols supplementation (WMD: 0.03 pg/mL, 95% CI: -1.51, 1.58, p = 0.966, Fig 3) using the random-effect model due to significant heterogeneity (I2 test = 81.6%, p = 0.001). Visual inspection of the funnel plot (S3 Fig), Egger’s test (p = 0.571), and Begg’s test (p = 1.000) indicated that there was no publication bias. The sensitivity analysis (S4 Fig) showed that omission of Daud et al. [19] or Stonehouse et al. [32] changed the direction of effect, but the result remained non-significant, suggesting that the finding was robust. Subgroup analysis was not performed for this outcome measure.
Fig 3. Forrest plot on the effect of tocotrienols supplementation on interleukin-6 levels.
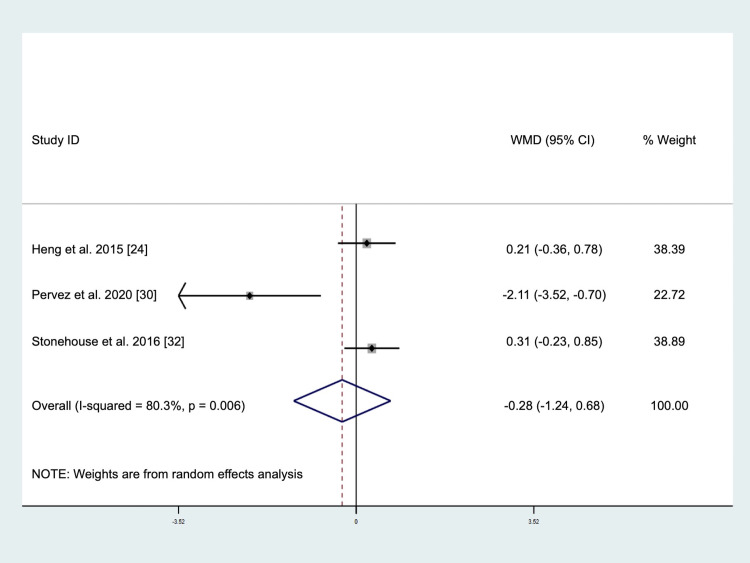
The pooled result of three studies involving a total of 185 subjects (intervention = 92, placebo = 93) demonstrated no significant changes in TNF-α levels after tocotrienols supplementation (WMD: -0.28 pg/mL, 95% CI: -1.24, 0.68, p = 0.571, Fig 4) using the random-effect model due to significant heterogeneity (I2 test = 80.3%, p = 0.006). Visual inspection of the funnel plot (S5 Fig) and Begg’s test (p = 0.117) indicated that there was no publication bias. Although Egger’s test (p = 0.011) was < 0.05, the trim-and-fill analysis did not identify any missing studies, suggesting a possible absence of bias. The sensitivity analysis (S6 Fig) showed that the omission of Pervez et al. [30] changed the direction of effect, but the result remained not significant, suggesting that the finding was robust. Subgroup analysis was not performed for this outcome measure.
Fig 4. Forrest plot on the effect of tocotrienols supplementation on tumor necrosis factor-alpha levels.
Effects of tocotrienols supplementation on oxidative stress markers
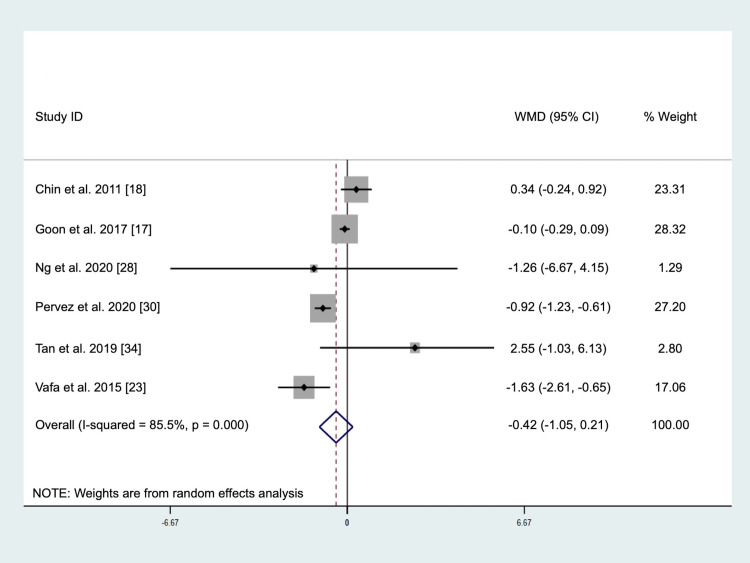
The pooled result of six studies involving a total of 356 subjects (intervention = 179, placebo = 177) demonstrated no significant changes in MDA levels after tocotrienols supplementation (WMD: -0.42 μmol/L, 95% CI: -1.05, 0.21, p = 0.189, Fig 5) using the random-effect model due to significant heterogeneity (I2 test = 85.5%, p < 0.001). Visual inspection of the funnel plot (S7 Fig), Egger’s test (p = 0.844), and Begg’s test (p = 0.573) indicated an absence of publication bias. The sensitivity analysis (S8 Fig) showed that omission of any study did not change the finding, suggesting that the result was robust. Subgroup analyses showed that the effect of tocotrienols on MDA levels was not influenced by study sample size or duration (Table 3). However, a significant reduction in MDA levels was observed in studies with tocotrienols dosage provided at least 400 mg/day (WMD: -0.90 μmol/L, 95% CI: -1.20, -0.59, p < 0.001).
Fig 5. Forrest plot on the effect of tocotrienols supplementation on malondialdehyde levels.
Table 3. Subgroup analyses of tocotrienols on malondialdehyde level.
| n | WMD (95% CI) | p within group | p heterogeneity | I2 (%) | |
|---|---|---|---|---|---|
| Study sample size | |||||
| ≥ 60 | 3 | -0.36 (-1.52, 0.80) | 0.546 | 0.001 | 86.0 |
| < 60 | 3 | -0.38 (-1.83, 1.06) | 0.602 | 0.004 | 82.2 |
| Study duration | |||||
| ≥ 6 months | 3 | -0.26 (-0.91, 0.40) | 0.444 | < 0.001 | 91.9 |
| < 6 months | 3 | 0.34 (-3.13, 2.46) | 0.812 | 0.087 | 59.0 |
| Dosage | |||||
| ≥ 400 mg/day | 3 | -0.90 (-1.20, -0.59) | < 0.001 | 0.165 | 44.6 |
| < 400 mg/day | 3 | -0.33 (-1.08, 0.42) | 0.393 | 0.003 | 82.7 |
Abbreviation: CI, confidence interval, WMD, weighted mean difference.
The effects of tocotrienols supplementation on oxidative stress markers are summarized in Table 4. Chin et al. [18] observed significant reductions in PC concentrations in subjects after receiving tocotrienols supplement (baseline: 0.63 ± 0.04 nmol/mg, 6th month: 0.45 ± 0.04 nmol/mg, p < 0.01), and subgroup analyses showed that the significant reduction was only observed among subjects ≥ 50 years old (baseline: 0.63 ± 0.03 nmol/mg, 6th month: 0.40 ± 0.03 nmol/mg, p < 0.01), but not 35–49 years old. Contrarily, Goon et al. [17] did not observe any significant change in PC concentrations in subjects receiving tocotrienols supplement (baseline: 0.44 ± 0.04 nmol/mg, 6th month: 0.33 ± 0.03 nmol/mg, p > 0.05).
Table 4. Descriptive summary of the effects of tocotrienols supplementation on oxidative stress markers, antioxidant enzyme activities and antioxidant response.
| Biomarkers | Summary of findings and reference |
|---|---|
| PC | ↓ [18], ↔ [17] |
| AGEs | ↔ [18,33] |
| TBARS | ↓ [25], ↔ [19] |
| LOPs | ↔ [25] |
| LDL oxidation rate | ↓ [29] |
| SOD | ↓ [18], ↑ [16] |
| CAT | ↔ [16,18] |
| GPx | ↔ [16,18] |
| GSH | ↔ [16] |
| Antioxidant response | ↑ TAS (320 mg/day) [31], ↔ TAP or TAC [19,23] |
Symbol: ↓, significant reduction, ↑, significant increase, ↔, no effect.
Abbreviations: AGEs, advanced glycation end-products, CAT, catalase, GPx, glutathione peroxidase, GSH, glutathione, LDL, low density lipoprotein, LOPs, lipid peroxidation products, PC, protein carbonyl, SOD, superoxide dismutase, TAC, total antioxidant capacity, TAP, total antioxidant power, TAS, total antioxidant status, TBARS, thiobarbituric acid reactive substances.
For AGEs, Chin et al. [18] showed that tocotrienols supplementation for 6 months did not lower serum AGE levels in all subjects (baseline: 2.38 ± 0.19 units/ml, 6th month: 1.92 ± 0.14 units/ml, p > 0.05), but a significant reduction in serum AGE level for subjects above 50 years old (baseline: 2.73 ± 0.26 units/ml, 6th month: 1.71 ± 0.24 units/ml, p < 0.05) was observed. On the other hand, Tan et al. [33] showed that changes in serum AGE levels were not significant between patients with type 2 diabetes mellitus receiving placebo or tocotrienols supplement (placebo: 83.7 ± 27.2 μg/mL, tocotrienols: 89.8 ± 27.2 μg/mL, p = 0.874).
Kooyenga et al. [25] reported significant reductions in TBARS levels in subjects after receiving tocotrienols (Baseline: 1.08 ± 0.14 μM, 24th month: 0.80 ± 0.14 μM, p < 0.05). Contrarily, Daud et al. [19] did not observe any significant change in TBARS levels with tocotrienols supplementations (baseline: 3.01 ± 4.65 μM MDA, 4th month: 2.89 ± 3.65 μM MDA, p > 0.05), though the tocotrienols group had a marginally (p = 0.055) lower trend in TBARS levels at the 4th month compared to the placebo group.
Kooyenga et al. [25] reported that the changes in LOPS levels were not significant in subjects after receiving tocotrienols supplementations (baseline: 1.73 ± 0.15 μM, 24th month 2.85 ± 0.55 μM, p > 0.05). Byrne et al. [29] compared the effects of three different tocotrienols isomers on LDL oxidative profile and showed significant reductions in the rate of LDL oxidation in subjects receiving α-tocotrienols (difference: -0.6 ± 0.9 nmol/mg/min, p <0.01) or δ-tocotrienols (difference: -0.1 ± 1.1 nmol/mg/min, p <0.06), but not γ-tocotrienols and placebo.
Effects of tocotrienols supplementation on antioxidant enzyme activities and response
The effects of tocotrienols supplementation on antioxidant enzyme activities and response are summarized in Table 4. There was a discrepancy in the finding of the effect of tocotrienols supplements on erythrocyte SOD levels [16,18], while no significant changes in erythrocyte CAT, GPx, and GSH levels following tocotrienols supplementation were observed. One study reported a significant increase in total antioxidant status with tocotrienols supplement [31], and this effect was not reported by the other two studies [19,23].
Risk of bias assessment
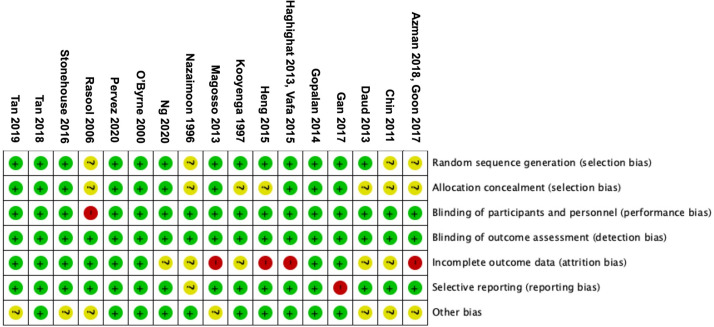
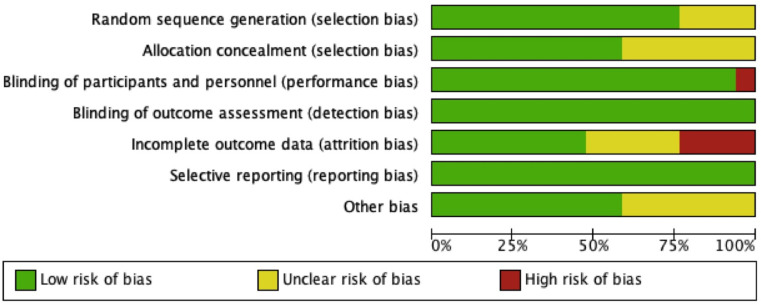
The bias assessment of the included studies is presented in Figs 6 and 7. A high risk of bias related to incomplete outcome data (attrition bias) was identified in six studies, which applied the “per-protocol” or “as-treated” analyses, while the other five studies had insufficient information to permit judgment. There were eight studies with unclear risk of selection bias concerning either random sequence generation or allocation concealment. One study had a blinded end-point design, which was considered high risk for performance bias. All studies were considered to have a low risk for detection bias as research personnels were blinded, and outcomes of this systematic review were objective markers and unlikely influenced by assessors. One study was considered to have a high risk for reporting bias due to a lack of baseline data. Seven studies had unclear risk of other biases, as there were potential risks of bias due to baseline characteristic imbalance or presence of potential confounding factors affecting the outcomes.
Fig 6. Risk of bias summary.
Fig 7. Risk of bias graph.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of randomized controlled trials to evaluate the effects of tocotrienols supplementation on circulating biomarkers of inflammation and oxidative stress. We demonstrated that tocotrienols supplementation reduced CRP levels. However, this finding needs to be interpreted with caution as the sensitivity analysis showed that the result was mainly driven by the study of Pervez et al. [30]. Once this study was excluded from the meta-analysis, the effects on CRP became non-significant. Pervez et al. [30] used an individual isoform of tocotrienols, namely δ-tocotrienols, while mixed tocotrienols were used by other studies [19–22,24,26,32]. In addition, the dosage of tocotrienols used by Pervez et al. [30] was 600 mg/day, while the remaining studies provided tocotrienols supplements with a dosage between 180 and 400 mg/day. Therefore, the type and dosage of tocotrienols supplementation may have contributed to the discrepancy of these findings as pooled analysis of studies supplementing mixed tocotrienols did not significantly lower CRP levels while only one study providing δ-tocotrienols showed a significant reduction in CRP levels. The subgroup analysis showed a significant reduction in CRP levels in studies with at least six months of intervention duration. For other markers, our meta-analyses demonstrated that tocotrienols supplementations had no effects on IL-6, TNF-α, and MDA levels, but the subgroup analysis showed that tocotrienols supplementation at 400 mg/day significantly lowered MDA levels. Therefore, it is suggested that future studies should consider at least providing tocotrienols supplement at 400 mg/day for six months.
Several meta-analyses have investigated the effects of vitamin E supplementation, particularly tocopherols, on inflammatory and oxidative stress outcomes. A meta-analysis of 12 studies by Saboori et al. [35] demonstrated that tocopherols supplementation was associated with significant reduction in CRP level by 0.62 mg/L (95% CI: -0.92, -0.31, p < 0.001). A meta-analysis of vitamin E supplementation (mainly tocopherols) in patients on hemodialysis also reported a significant reduction in CRP levels [36]. Similarly, a recent meta-analysis inclusive of studies investigating both tocopherols and tocotrienols showed a significant reduction in CRP levels, while the significant reductions in IL-6 and TNF-α were reported for α-tocopherol and γ-tocopherol, respectively [12]. It must be noted that a subgroup analysis for tocotrienols was not performed, and several studies on tocotrienols [22,24,26,30] were not included in the meta-analysis by Asbaghi et al. [12]. Contrarily, Fouladvand et al. [37] pooled the results from eight studies on vitamin C and E co-supplementation and showed no significant effect on CRP levels. For oxidative stress, Sepidarkish et al. [38] showed that omega-3 fatty acids and vitamin E co-supplementation reduced MDA levels and increased total antioxidant capacity and nitric oxide levels. However, no significant effects on GSH, CAT, and SOD were observed. The meta-analysis by Rad et al. [39] showed that vitamin E supplementation significantly reduced plasma isoprostane level but had no effect on urine isoprostane level. It is important to highlight that these findings were primarily based on tocopherols and should not be extrapolated to tocotrienols.
Pre-clinical studies have proposed several mechanisms for the anti-inflammatory properties of tocotrienols: (i) inhibition of NF-kB [40], (ii) suppressing the expression of inflammatory mediators such as TNF-α, interleukin-1, IL-6, interleukin-8, inducible nitric oxide synthase, and (iii) suppressing the STAT3 cell-signaling pathway [10]. Several preclinical studies have investigated the anti-inflammatory properties of individual tocotrienols isoforms. Yam et al. [41] compared the anti-inflammatory effects of tocotrienols rich fraction (TRF) and individual tocotrienols isoforms using the model of lipopolysaccharide (LPS)-stimulated RAW264.6 macrophages. It was demonstrated that the δ-tocotrienols had the highest inhibitory effect on the production of IL-6, followed by γ-tocotrienols, α-tocotrienols, and TRF. On the other hand, production of TNF-α was reduced in the α-tocotrienols group, unaltered in the γ-tocotrienols, and increased in the TRF and δ-tocotrienols groups. On the contrary, Qureshi et al. [42] demonstrated that δ-tocotrienols had the greatest inhibitory effect on TNF-α in LPS-stimulated RAW 264.7 cells and 6-week old female BALB/c mice, followed by γ-tocotrienols and α-tocotrienols. Muid et al. [43] showed that both δ- and γ-tocotrienols had a higher potency in terms of inhibiting IL-6 production and NF-κB activation in LPS-stimulated human umbilical vein endothelial cells than α- and β-tocotrienols. Selvaduray et al. [44] also reported that the capacity of suppressing the production of IL-6 and IL-8 in human umbilical vein endothelial cells was most significant in δ-tocotrienols, followed by γ-tocotrienols and TRF. Nishio et al. [45] showed that both the capacity of α- and γ-tocotrienols suppressing the production of reactive oxygen species and expression of TNF-α and IL-8 induced by treatment of LPS to human lung carcinoma A49 cells was not significantly different. Therefore, individual isoforms of tocotrienols have different potency of anti-inflammatory effects, and it is critical to distinguish these isomers when interpreting the findings of studies included in this systematic review.
However, the present systematic review of clinical trials could not provide conclusive clinical evidence on the anti-inflammatory and antioxidant effects of tocotrienols. Similarly, despite the accumulating evidence from in vitro and animal studies suggesting that tocotrienols are a potential hypocholesterolemic agent [9], a recent meta-analysis by Zuo et al. [46] also demonstrated that tocotrienols supplementation only significantly increased high-density lipoprotein-cholesterol levels but had no influence on total cholesterol, low-density lipoprotein cholesterol, or triglyceride levels. Therefore, it appeared that findings from pre-clinical studies were not entirely reproducible in human trials. Several possibilities on the challenges of clinical translation from bench to bedside merit consideration. Firstly, tocotrienols have relatively low bioavailability, and the oral absorption of tocotrienols is highly dependent on the consumption of dietary fat due to their lipid-solubility, thus ensuring bile secretion and emulsification process in the intestine to facilitate absorption [9]. Orally administered tocotrienols have shorter elimination half-time by 4.5–8.7 times compared to α-tocopherol [47]. Therefore, twice-daily dosing of tocotrienols supplement is necessary to ensure tocotrienols in the circulating plasma for a sustained period [48]. In addition, supplementation dose and formulation, subjects’ age, and underlying diseases are critical factors affecting the bioavailability and clinical efficacy of tocotrienols [49]. In the present systematic review, eight studies [16–18,21,26,29,31,32] reported a significant increase in serum tocotrienols levels, while one study reported a non-significant increase in tocotrienols level [27]. The remaining studies did not assess changes in serum tocotrienols levels.
The present systematic review included randomized controlled trials reporting a wide variety of biomarkers of inflammation, oxidative stress, and antioxidant enzyme and response with very limited common parameters for meta-analysis. The most frequently reported inflammatory biomarkers were CRP, IL-6, and TNF-α, while MDA was the most frequently reported oxidative stress marker. Inflammation and oxidative stress are biological responses orchestrated via a complex network of interactions, and these four biomarkers only represent a small part of these processes. In addition, these markers do not differentiate acute and chronic inflammation, and different phases of inflammatory responses. Measurement of isoprostanes with the mass spectrometric technique is known as the standard of oxidative stress assessment [50], yet none of the studies included in this systematic review reported it. Contrarily, antioxidant enzymes were commonly reported, albeit these markers reflected neither inflammation nor oxidative stress. Robust and validated markers are essential considerations for human nutritional studies elucidating the role of nutrition supplements such as tocotrienols in inflammation and oxidative stress. Therefore, a cluster of validated markers instead of a single marker has been recommended to evaluate inflammatory and oxidative stress status [51]. The use of new omics technologies could also shed light on the impacts of tocotrienols supplementation on inflammation and oxidative stress.
It is also important to note that these inflammatory and oxidative stress markers may fluctuate over time, even in healthy individuals, as several modifying factors influence the concentrations of these markers, including age, body fatness, physical activity, sex, genotype, smoking habits, diet, the composition of the gut microbiota and the use of certain medications [51]. For instance, individuals with chronic diseases may exhibit a greater degree of chronic inflammation than healthy individuals. In this systematic review, we included a heterogeneous group of subjects with different disease statuses. Therefore, the effective dose of tocotrienols to exert anti-inflammatory and antioxidative effects may vary by subjects’ characteristics and underlying conditions, which leads to null findings in some analyses. In the context of dietary intake, most studies included in this systematic review were conducted in Malaysia, and about 84–90% of Malaysians regularly consume palm oil [52,53], which contains a high amount of tocotrienols (940 mg/kg) [10]. Therefore, baseline and variations in dietary habits of these subjects should be taken into consideration.
Our meta-analysis had several limitations. Firstly, although 19 studies were included in the present systematic review, the number of studies analyzing specific biomarkers was small. We could not perform meta-analysis if fewer than three studies reported these oxidative stress markers and antioxidant enzyme activities. Although we performed meta-analyses for four biomarkers of inflammation and oxidative stress, these selected biomarkers do not represent the whole picture of the complex physiological process of inflammatory response and oxidative stress. Secondly, most studies had a relatively small sample size and short intervention period. Thirdly, we could not perform subgroup analyses for meta-analyses on IL-6 and TNF-α due to limited studies; therefore, the source of between-study heterogeneity cannot be identified. In addition, potential bias may exist in the group selection for the subgroup analyses. However, the subgroup analyses aimed to generate a hypothesis on the potential effects of tocotrienols based on the study characteristics that require further investigation. Fourthly, some studies may be underpowered, as markers of inflammation and oxidative stress were not designated as the primary outcome. Then, studies included in this systematic review consisted of subjects with varied underlying diseases associated with a different degree of inflammation and oxidative stress. Lastly, the external validity of our results requires further confirmation as most studies were conducted in Malaysia.
Conclusions
The meta-analysis showed that tocotrienols supplementations significantly lowered CRP levels, but this finding was primarily attributed to a single study using δ-tocotrienols, not mixed tocotrienols. In addition, there were no significant effects on IL-6, TNF-α, and MDA levels. Although it appeared that a higher dose of tocotrienols (≥ 400 mg/day) might reduce MDA levels, further studies are required to confirm this observation. The effects of tocotrienols supplementations on other oxidative stress markers, antioxidant enzyme activities and status were inconclusive due to limited high-quality clinical evidence. More randomized controlled trials are warranted to confirm the anti-inflammatory and antioxidant effects of tocotrienols by assessing validated inflammatory and oxidative stress biomarkers determined using state-of-the-art technologies. In addition, the effects of different isoforms and dosages of tocotrienols, and populations that are most likely to benefit from the supplementation, such as populations with suboptimal baseline plasma tocotrienols concentration or known active inflammatory diseases, should also be investigated.
Supporting information
(DOC)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We appreciate the authors who generously shared their data for the meta-analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study is funded by Universiti Kebangsaan Malaysia (https://research.ukm.my) to AHAG (MI-2020-004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008; 454 (7203): 428–435. doi: 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 2.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015; 114 (7): 999–1012. doi: 10.1017/S0007114515002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017; 2017: 8416763. doi: 10.1155/2017/8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016; 2016: 5698931. doi: 10.1155/2016/5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019; 25 (12): 1822–1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020; 11: 694. doi: 10.3389/fphys.2020.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front Pharmacol. 2017; 8: 444. doi: 10.3389/fphar.2017.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Q. Natural forms if vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014; 72: 76–90. doi: 10.1016/j.freeradbiomed.2014.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SK, Kamisah Y, Mohamed N, Muhammad N, Masbah N, Fahami NAM, et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients. 2020; 12 (1): 259. doi: 10.3390/nu12010259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab. 2014; 11: 52. doi: 10.1186/1743-7075-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nesaretnam K, Meganathan P. Tocotrienols: inflammation and cancer. Ann N Y Acad Sci. 2011; 1229 (1): 18–22. doi: 10.1111/j.1749-6632.2011.06088.x [DOI] [PubMed] [Google Scholar]
- 12.Asbaghi O, Sadeghian M, Nazarian B, Sarreshtedari M, Mozaffari-Khosravi H, Maleki V, et al. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. 2020; 10(1): 1–17. doi: 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009; 6: e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadic AJ, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol. 2016; 74: 119–123. doi: 10.1016/j.jclinepi.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azman NHEN, Goon JA, Abdul Ghani SM, Hamid Z, Wan Ngah WZ. Comparing Palm Oil, Tocotrienol-Rich Fraction and α-Tocopherol Supplementation on the Antioxidant Levels of Older Adults. Antioxidants. 2018; 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goon JA, Azman NHEN, Ghani SMA, Hamid Z, Ngah WZW. Comparing palm oil tocotrienol rich fraction with α-tocopherol supplementation on oxidative stress in healthy older adults. Clin Nutr ESPEN. 2017; 21: e1–e12. doi: 10.1016/j.clnesp.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Chin SF, Ibahim J, Makpol S, Hamid NAA, Latiff AA, Zakaria Z, et al. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr Metab. 2011; 8: 42. doi: 10.1186/1743-7075-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daud ZAM, Tubie B, Sheyman M, Osia R, Adams J, Tubie S, et al. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc Health Risk Manag. 2013; 9: 747–761. doi: 10.2147/VHRM.S51710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan YL, Fu JY, Lai OM, Chew BH, Yuen KH, Teng KT, et al. Effect of palm-based tocotrienols and tocopherol mixture supplementation on platelet aggregation in subjects with metabolic syndrome: a randomised controlled trial. Sci Rep. 2017; 7: 11542. doi: 10.1038/s41598-017-11813-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopalan Y, Shuaib IL, Magosso E, Ansari MA, Abu Bakar MR, Wong JW, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke. 2014; 45: 1422–1428. doi: 10.1161/STROKEAHA.113.004449 [DOI] [PubMed] [Google Scholar]
- 22.Haghighat N, Vafa M, Eghtesadi S, Heidari I, Hosseini A, Rostami A. The effects of tocotrienols added to canola oil on microalbuminuria, inflammation, and nitrosative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Int J Prev Med. 2014; 5: 617–623. [PMC free article] [PubMed] [Google Scholar]
- 23.Vafa M, Haghighat N, Moslehi N, Eghtesadi S, Heydari I. Effect of Tocotrienols enriched canola oil on glycemic control and oxidative status in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. J Res Med Sci. 2015: 20: 540–547. doi: 10.4103/1735-1995.165945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heng KS, Hejar A, Johnson SJ, Ooi CP, Loh SP. Potential of Mixed Tocotrienol Supplementation to Reduce Cholesterol and Cytokines Level in Adults with Metabolic Syndrome. Mal J Nutr. 2015; 22: 231–243. [Google Scholar]
- 25.Kooyenga DK, Geller M, Watkins TR, Gapor A, Diakoumakis E, Bierenbaum ML. Palm oil antioxidant effects in patients with hyperlipidaemia and carotid stenosis-2 year experience. Asia Pac J Clin Nutr. 1997; 6 (1): 72–75. [PubMed] [Google Scholar]
- 26.Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NAK, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013; 12: 166. doi: 10.1186/1475-2891-12-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nazaimoon WW, Sakinah O, Gapor A, Khalid BAK. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr Res. 1996; 16 (11–12): 1901–1911. [Google Scholar]
- 28.Ng YT, Phang SCW, Tan GCJ, Ng EY, Botross Henien NP, M Palanisamy UD, et al. The effects of tocotrienol-rich vitamin E (Tocovid) on diabetic neuropathy: a phase II randomized controlled trial. Nutrients. 2020; 12: 1522. doi: 10.3390/nu12051522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Byrne D, Grundy S, Packer L, Devaraj S, Baldenius K, Hoppe PP, et al. Studies of LDL oxidation following α-, γ-, or δ-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic Biol Med. 2000; 29: 834–845. doi: 10.1016/s0891-5849(00)00371-3 [DOI] [PubMed] [Google Scholar]
- 30.Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled trial. Complement Ther Med. 2020; 52: 102494. doi: 10.1016/j.ctim.2020.102494 [DOI] [PubMed] [Google Scholar]
- 31.Rasool AH, Yuen KH, Yusoff K, Wong AR, Rahman AR. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol. 2006; 52: 473–478. doi: 10.3177/jnsv.52.473 [DOI] [PubMed] [Google Scholar]
- 32.Stonehouse W, Brinkworth GD, Thompson CH, Abeywardena MY. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis. 2016; 254: 205–214. doi: 10.1016/j.atherosclerosis.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 33.Tan SMQ, Chiew Y, Ahmad B, Kadir KA. Tocotrienol-rich vitamin E from palm oil (tocovid) and its effects in diabetes and diabetic nephropathy: a pilot phase II clinical trial. Nutrients, 2018; 10: 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan GCJ, Tan SMQ, Phang SCW, Ng YT, Ng EY, Ahmad B, et al. Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial. Ther Adv Endocrinol Metab. 2019; 10: 1–16. doi: 10.1177/2042018819895462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saboori S, Shab-Bidar S, Speakman JR, Rad EY, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2015; 69 (8): 867–873. doi: 10.1038/ejcn.2014.296 [DOI] [PubMed] [Google Scholar]
- 36.Khor BH, Narayanan SS, Sahathevan S, Gafor AHA, Daud ZAM, Khosla P, et al. Efficacy of nutritional interventions on inflammatory markers in haemodialysis patients: a systematic review and limited meta-analysis. Nutrients. 2018; 10: 397. doi: 10.3390/nu10040397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouladvand F, Falahi E, Asbaghi O, Abbasnezhad A. Effect of vitamins C and E co-supplementation on serum C-reactive protein level: a systematic review and meta-analysis of randomized controlled trials. Prev Nutr Food Sci. 2020; 25: 1–8. doi: 10.3746/pnf.2020.25.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sepidarkish M, Akbari-Fakhrabadi M, Daneshzad E, Yavari M, Rezaeinejad M, Morvaridzadeh M, et al. Effect of omega-3 fatty acid plus vitamin E so-supplementation on oxidative stress parameters: a systematic review and meta-analysis. Clin Nutr. 2020; 39: 1019–1025. doi: 10.1016/j.clnu.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 39.Rad EY, Falahi E, Djalali M, Abbasnezhad A, Birjandi M, Saboori S. Effect of vitamin E supplementation on plasma and urine levels of isoprostane F2α in randomized controlled clinical trials: a systematic review and meta-analysis. Int J Vitam Nutr Res. 2018; 87: 314–321. [DOI] [PubMed] [Google Scholar]
- 40.Kaileh M, Sen R. Role of NF-κB in the anti-inflammatory effects of tocotrienols. J Am Coll.Nutr. 2010; 29: 334S–339S. doi: 10.1080/07315724.2010.10719848 [DOI] [PubMed] [Google Scholar]
- 41.Yam ML, Hafid ARA, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009; 44: 787–797. doi: 10.1007/s11745-009-3326-2 [DOI] [PubMed] [Google Scholar]
- 42.Qureshi AA, Reis JC, Papasian CJ, Morrison DC, Qureshi N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis. 2010; 9: 143. doi: 10.1186/1476-511X-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muid A, Froemming GRA, Rahman T, Ali AM, Nawawi HM. Delta- and gamma- tocotrienol isomers are potent in inhibiting inflammation and endothelial activation in stimulated human endothelial cells. Food Nutr Res. 2016; 60: 31526. doi: 10.3402/fnr.v60.31526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretnam K. Palm tocotrienols decrease levels of pro-angiogenic markers in human unbilical vein endothelial cells (HUVEC) and murine mammary cancer cells. Genes Nutr. 2021; 7:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishio K, Horie M, Akazawa Y, Shichiri M, Iwahashi H, Hagihara Y, et al. Attenuation of lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biol. 2013; 1: 97–103. doi: 10.1016/j.redox.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuo S, Wang G, Han Q, Xiao H, Santos HO, Rodriguez DA, et al. The effects of tocotrienol supplementation on lipid profile: A meta-analysis of randomized controlled trials. Complement Ther Med. 2020; 52: 102450. doi: 10.1016/j.ctim.2020.102450 [DOI] [PubMed] [Google Scholar]
- 47.Zhao L, Fang X, Marshall MR, Chung S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules. 2016; 21: 344. doi: 10.3390/molecules21030344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meganathan P, Fu JY. Biological properties of tocotrienols: evidence in human studies. Int J Mol Sci. 2016; 17: 1682. doi: 10.3390/ijms17111682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu JY, Che HL, Tan DMY, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutr Metab. 2014; 11: 5. doi: 10.1186/1743-7075-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho E, Galougahi KK, Liu CC, Bhindi R, Figtree G. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013; 1(1): 483–491. doi: 10.1016/j.redox.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr. 2013; 109: S1–S34. [DOI] [PubMed] [Google Scholar]
- 52.Khor BH, Sahathevan S, Sualeheen A, Ali MSM, Narayanan SS, Chinna K, et al. Dietary fatty acid intake in hemodialysis patients and associations with circulating fatty acid profiles: A cross-sectional study. Nutrition. 2019; 63: 14–21. doi: 10.1016/j.nut.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 53.Karupaiah T, Chuah KA, Chinna K, Pressman P, Clemens RA, Hayes AW, et al. A cross-sectional study on the dietary pattern impact on cardiovascular disease biomarkers in Malaysia. Sci Rep. 2019; 9: 13666. doi: 10.1038/s41598-019-49911-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.