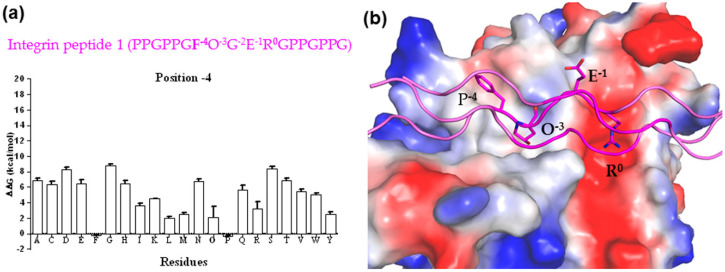
Figure 3.
(a) Predicted total folding energies for the substitution of residues at position -4 in integrin peptide 1 (PPGP−7P−6GF−4O−3GE−1R0GPPGPPG) and the HSP47 complex compared to that of WT (ΔΔG ± SD, n = 5, kcal/mol). The lower energy indicates favorable binding. (b) The predicted binding mode between triple-helical integrin peptide 1 (shown as magenta cartoon and sticks) and HSP47 (shown on electrostatic potential surface).