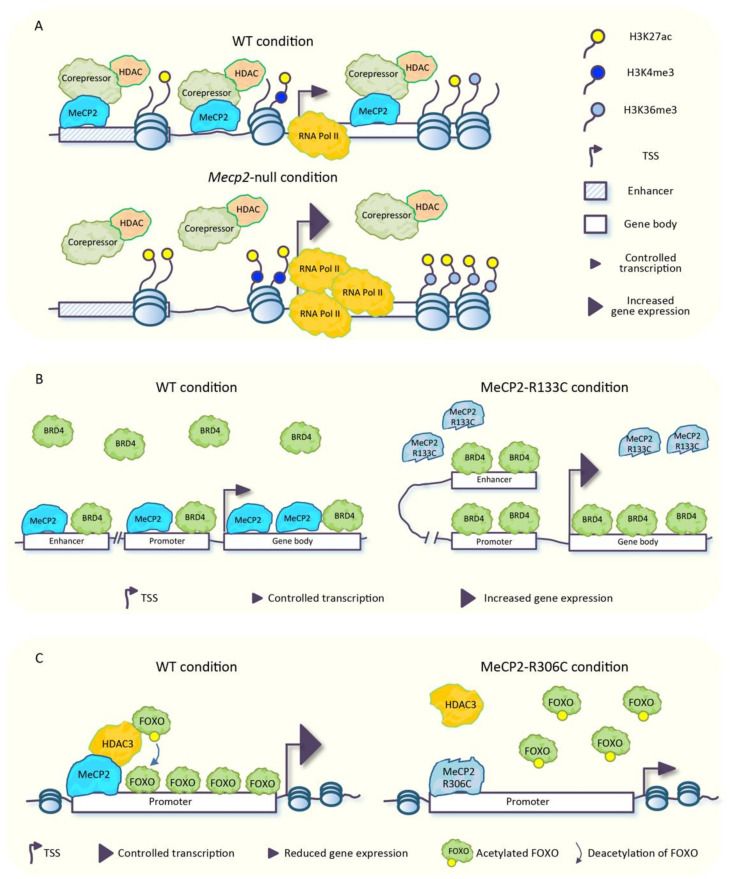
Figure 2.
MeCP2 dysfunctions lead to uncontrolled distribution of epigenetic factors and histone modifications. (A) In WT neurons, MeCP2 controls gene expression by recruiting complexes containing histone deacetylase activity, which survey the acetylation status of H3K27 across enhancers, TSS, and gene bodies (top). The absence of MeCP2 leads to the enhanced acetylation of H3K27 across regulatory regions and gene body and causes a higher accumulation of RNA pol II at TSS. These changes are accompanied by a higher enrichment of H3K4me3 at TSS and of H3K36me3 at gene bodies, which correlate with gene up-regulation (bottom) (adapted from [61,83]). (B) In WT interneurons, in basal physiological conditions, the binding of BRD4 across enhancers, promoters, and gene bodies is low (left); in MeCP2-R133C interneurons, the binding of BRD4 across gene bodies and regulatory regions increases. These changes parallel a higher frequency of enhancer/promoter interactions with the resulting gene up-regulation (adapted from [84,85]). (C) In physiological conditions, MeCP2 recruits HDAC3, which deacetylates FOXO transcription factors [86]. Once deacetylated, FOXO proteins bind DNA and activate transcription (left) [87,88]. In MeCP2-R306C neurons, the increased acetylation of FOXO proteins occurs, which leads to the reduced enrichment of these transcription factors to promoters. These events lead to gene down-regulation (right) [86].