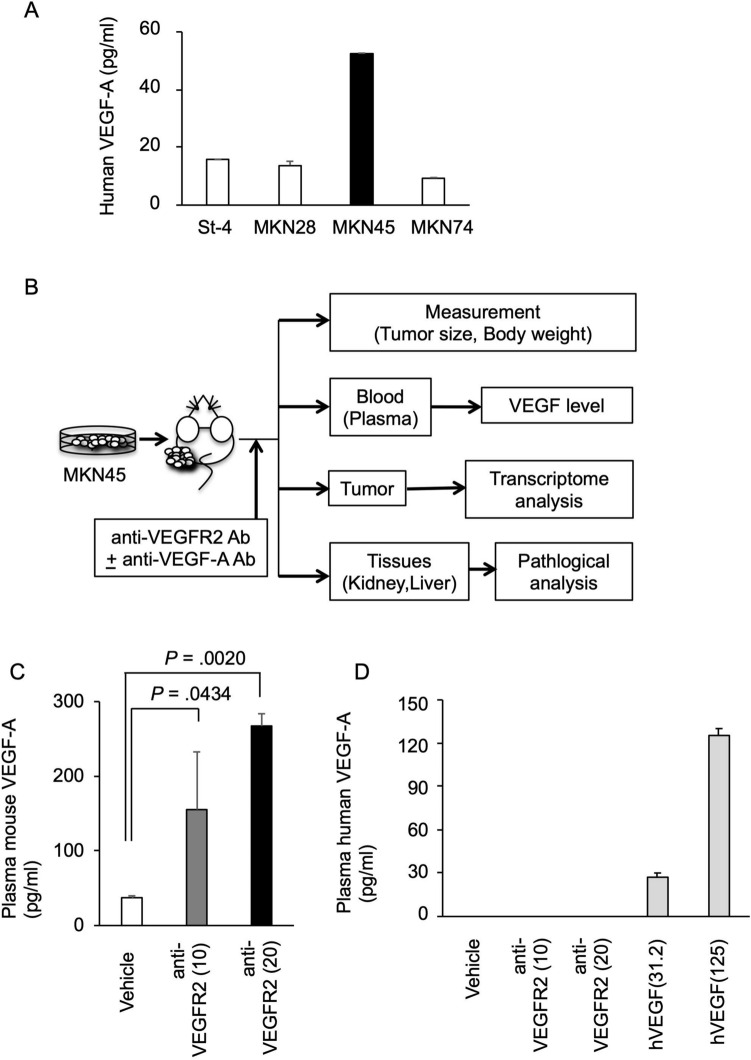
Figure 1.
Elevated host-derived VEGF-A in the blood after VEGFR2-targeted therapy in a gastric cancer xenograft model. (A) Human VEGF-A levels secreted by human gastric cancer cell lines. Human gastric cancer cell lines were cultured for 72 h, and conditioned media were collected. Human VEGF-A level in the conditioned medium was measured as described in Materials and Methods. The VEGF-A concentrations normalized by cell number (per 1 × 104 cells) are shown. (B) Schematic diagram of the evaluation of plasma VEGF levels and therapeutic effect of VEGFR2 and VEGF-A dual targeting in a gastric cancer xenograft model. (C,D) Alterations in murine (C) and human (D) VEGF-A levels in mouse plasma following anti-mouse VEGFR2 antibody administration were shown. BALB/c nude mice were subcutaneously injected with 3 × 106 MKN45 cells. When the tumor reached 100–200 mm3, mice were intraperitoneally treated with vehicle (PBS) or an anti-mouse VEGFR2 antibody (DC101) (10 or 20 mg/kg) twice a week for 2 weeks (N = 3). At 14 days after the start of treatment, mouse plasma was collected, and human and murine VEGF-A concentrations were measured as described in Materials and Methods. For the estimation of human VEGF-A levels, purified human VEGF-A protein solutions (hVEGF, 31.2 and 125 pg/ml) were also tested as controls. Statistical significance was evaluated by ANOVA, followed by the Tukey–Kramer post-hoc test. The figures were generated by Microsoft Powerpoint (16.16.27) (https://www.microsoft.com/ja-jp/microsoft-365/powerpoint).