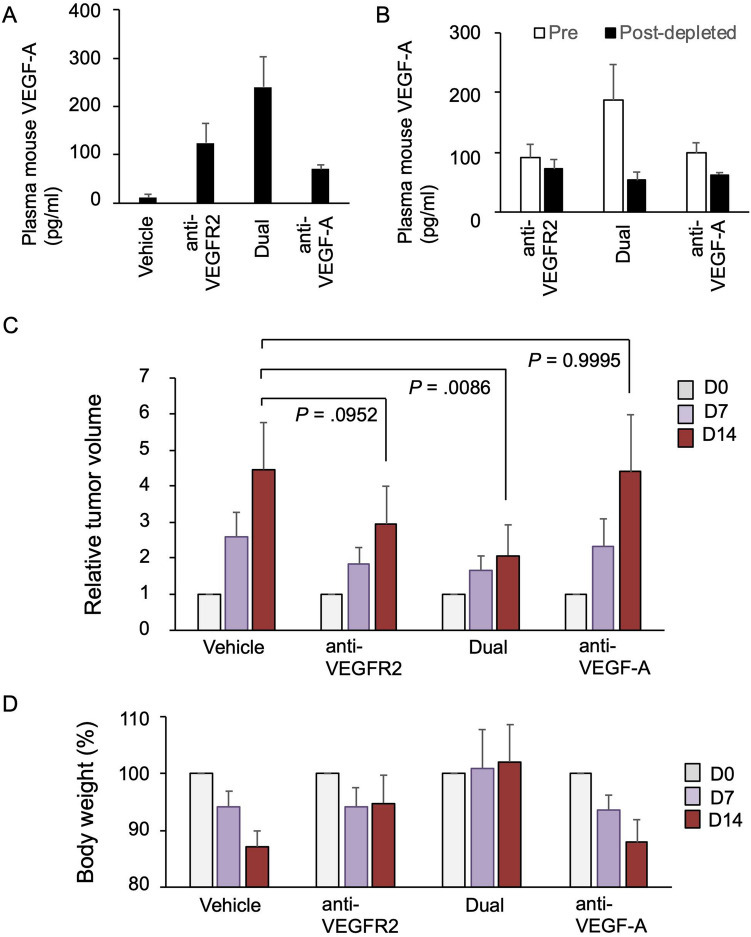
Figure 2.
Effect of the co-administration of a VEGF-A neutralizing antibody and VEGFR2-targeted antibody in a gastric cancer xenograft model. (A) Plasma VEGF-A levels after anti-VEGFR2 antibody and/or anti-VEGF-A antibody treatment. BALB/c nude mice were injected with MKN45 cells. When the tumor reached 100–200 mm3, the mice were intraperitoneally treated with vehicle (PBS), an anti-mouse VEGFR2 antibody (10 mg/kg), an anti-mouse VEGF-A antibody (5 mg/kg), and the two antibodies together (Dual) twice a week for 2 weeks (N = 3). At 14 days after the start of treatment, mouse plasma was collected, and the murine VEGF-A concentration was measured. (B) Comparison of total and free VEGF-A (unbound to antibody) levels in the plasma of mice treated with the anti-VEGFR2 antibody alone or together with the anti-VEGF-A antibody (Dual). BALB/c nude mice were injected with MKN45 cells, treated with the anti-mouse VEGFR2 antibody (10 mg/kg) alone or together with the anti-mouse VEGF-A antibody, and murine plasma was collected as in (A). The plasma was incubated in the presence or absence of protein-G beads to deplete the antibody-bound fraction, and mouse VEGF-A levels were measured as described in Materials and Methods. (C,D) BALB/c nude mice were injected with MKN45 cells. When the tumor reached 100–200 mm3, the mice were treated with the vehicle (N = 7), anti-VEGFR2 antibody (10 mg/kg) alone (N = 8), anti-VEGFR2 antibody (10 mg/kg) and anti-VEGF-A antibody (5 mg/kg) together (Dual, N = 6), and anti-VEGF-A antibody (5 mg/kg) alone (N = 5). The length (L) and width (W) of each tumor was measured at days 0, 7, and 14 using a digital caliper, and the tumor volume was calculated as (L × W2)/2. Relative tumor volume was shown in (C). Statistical significance was estimated by ANOVA, followed by the Tukey–Kramer post-hoc test. Body weight changes in mice in the treatment groups were indicated in (D). The figures were generated by Microsoft Powerpoint (16.16.27) (https://www.microsoft.com/ja-jp/microsoft-365/powerpoint).