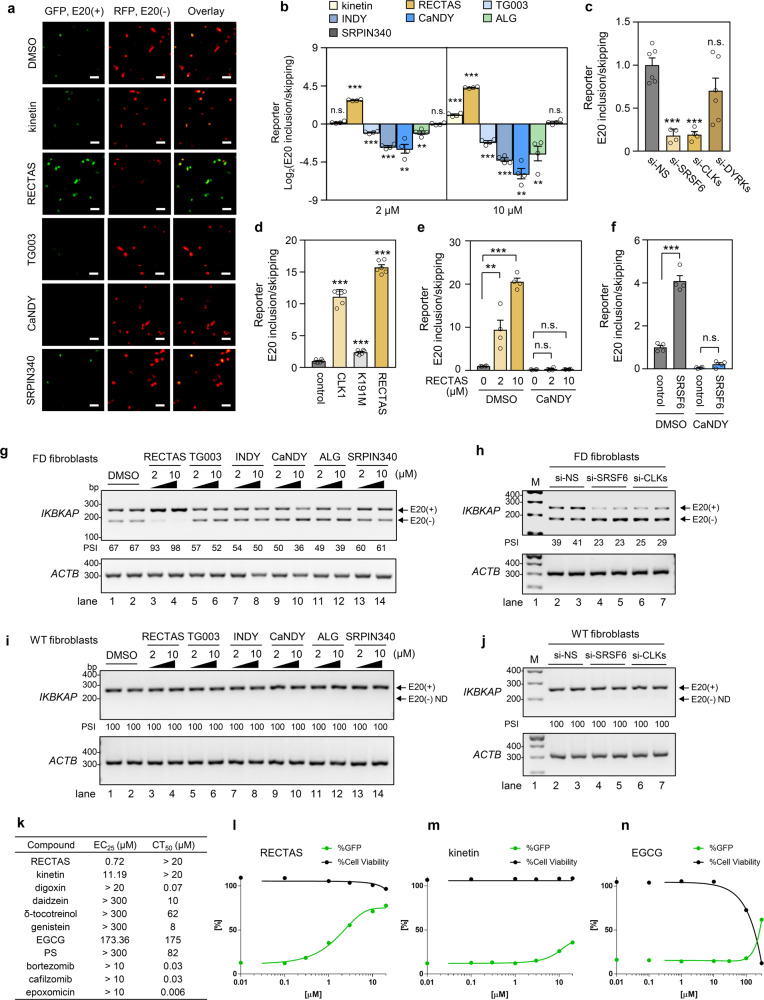
Fig. 2. A pivotal control of IKBKAP-FD exon 20 splicing by RECTAS and a CLK inhibitor.
a, b Microscopic images (a) and IKBKAP-FD exon 20 inclusion rates (b) following compound treatment (2 or 10 µM) or solvent only (0.1% DMSO) for 24 h in HeLa cells transfected with IKBKAP-FD reporter. ALG indicates ALGERNON in (b). c HeLa cells with IKBKAP-FD reporter were transfected with siRNA for si-SRSF6, si-CLKs, si-DYRKs, or si-NS for 72 h, and IKBKAP-FD exon 20 inclusion rates were plotted by GFP/RFP ratio. d IKBKAP-FD exon 20 inclusion was quantified by the GFP/RFP ratio of IKBKAP-FD reporter in HeLa cells, transfected with empty vector (control), CLK1 expression vector, or inactive mutant CLK1 (K191M) expression vector for 24 h, or treated with RECTAS (2 µM) for 24 h. e, f IKBKAP-FD exon 20 inclusion rates in response to RECTAS treatment for 24 h (e) or transfection with SRSF6 expression vector for 24 h (f) were quantified by GFP/RFP ratio in HeLa cells, transfected with IKBKAP-FD reporter with or without CaNDY treatment (10 µM, 24 h). control, empty p3XFLAG-CMV14 vector transfection in (f). g, h RT-PCR for IKBKAP-FD exon 19–21 for FD patient fibroblasts (P2) treated with compounds (2 or 10 µM) or solvent only (0.1 % DMSO) for 24 h (g), or transfected with si-NS, si-SRSF6, or si-CLKs for 72 h (h). i, j RT-PCR for IKBKAP-FD exon 19–21 for fibroblasts from a healthy individual (C1) treated with compounds (2 or 10 µM) or solvent only (0.1% DMSO) for 24 h (i), or transfected with si-NS, si-SRSF6, or si-CLKs for 72 h (j). k–n 25% effective concentration (EC25) and 50% cytotoxic concentration (CT50 for 72 h treatment) were shown for indicated compounds. EC25 was determined with %GFP of 20 µM RECTAS set to 100%. Individual plots for RECTAS (l), kinetin (m), and (−)-epigallocatechin gallate (EGCG) (n) are shown separately. Data from four replicates are shown in (a), (b), (c), (e), and (f), six replicates in (d), four replicates for %GFP, and three replicates for %Cell viability in (k–n). Representative data from two replicates are shown in (g–j). Columns, mean; bars, SE; n.s., P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001 for unpaired two-tailed t test in (b) (vs. the 0.1% DMSO control) (kinetin: 2 µM, P = 9.2 × 10−2; 10 µM, P = 8.8 × 10−5; RECTAS: 2 µM, P = 3.2 × 10−8; 10 µM, 3.2 × 10−9; TG003: 2 µM, 2.3 × 10−5; 10 µM, 1.3 × 10−5; INDY: 2 µM, 1.5 × 10−6; 10 µM, 1.6 × 10−6; ALG: 2 µM, P = 4.1 × 10−3; 10 µM, P = 7.1 × 10−3; SRPIN340: 2 µM, P = 0.90; 10 µM, P = 0.27), (c) (vs. si-NS: si-SRSF6, P = 6.7 × 10−5; si-CLKs, P = 7.1 × 10−5; si-DYRKs, P = 0.11), (d) (vs. control vector: CLK1, P = 1.1 × 10−9; K191M, P = 1.0 × 10−5; RECTAS, P = 6.1 × 10−12), (e) (P = 9.0 × 10−3 and P = 3.9 × 10−7 for RECTAS 2 µM and 10 µM without CaNDY, and P = 0.53 and 0.13 for RECTAS 2 µM and 10 µM with CaNDY), and (f) (DMSO, P = 3.1 × 10−5; CaNDY, P = 8.0 × 10−2). E20 (+), exon 20 inclusion product; E20 (−), exon 20 skipping product in (a), (g), (h), (i), and (j); ND, not detectable in (i) and (j); PSI, percent spliced-in in (g–j). IKBKAP and ACTB were detected with primers oAM138 + oAM139 and oAM13 + oAM14, respectively in (g–j).