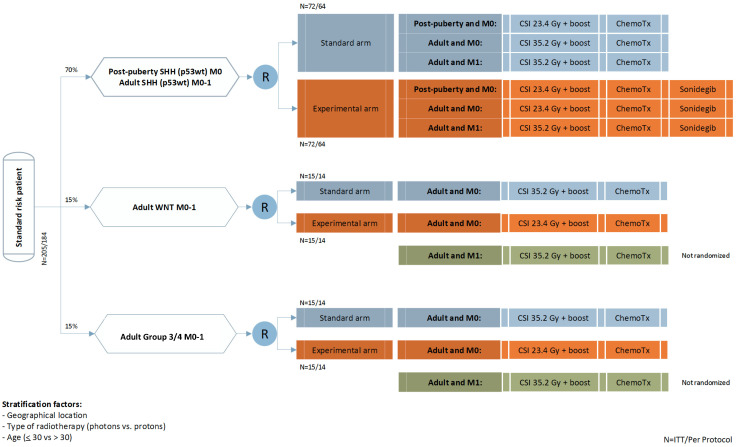
Figure 1.
Trial design: Patients will be stratified into their respective genetic subgroups. Randomization will be performed into regular-dose (35.2 Gy) and low-dose (23.4 Gy) radiotherapy for all strata, and radio-chemotherapy vs. reduced radio-chemotherapy plus sonidegib in the SHH subgroup. Chemotherapy consists of 4 doses of vincristine 1.5 mg/m2 (maximum 2 mg) during radiotherapy, followed by a maximum of 6 cycles of lomustine 75 mg/m2 on day 1, cisplatin 70 mg/m2 on day 1, and vincristine 1.5 mg/m2 (max. 2 mg) on day 1 and 15 of 6-weekly cycles. The following modification for post-pubertal patients aged 17 and below with WNT and group 3/group 4 will apply: these patients will not be treated in the EORTC 1634-BTG/NOA-23 trial and will be recommended to participate in a suitable pediatric trial. N = number of patients; R = randomization; SHH, WNT, group 3/group 4 are medulloblastoma subgroups; M = metastasis; CSI = craniospinal irradiation; Gy = gray; ChemoTx = chemotherapy, ITT = intent to treat. Please note that group 3 patients were not included in the initial version of the protocol, but will be included in the amended version 3.0 as depicted.