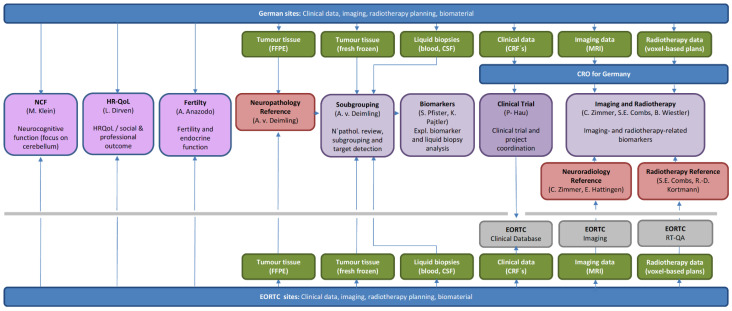
Figure 2.
Graphic representation of the overall trial and translational project organization: EORTC is the sponsor of the trial, will host the trial in cooperation with Neuro-Onkologische Arbeitsgemeinschaft in der Deutschen Krebsgesellschaft (NOA), and ensure processing of clinical data, imaging data, and biomaterial that are analyzed within the translational subprojects. Reference sites for neuropathology, neuroimaging, and radiotherapy quality assurance have been defined and will directly receive biomaterial from sites and MRI images and voxel-based radiotherapy plans from the EORTC imaging platform and EORTC quality assurance in radiotherapy (RT-QA) platform. Neuro-cognition (NCF), health-related quality of life (HRQoL), and fertility and endocrine function will be assessed centrally. Responsible sites and principal investigators are named in the respective task boxes. WP = work package; FFPE = formalin-fixed paraffin-embedded; CSF = cerebrospinal fluid; CRF = case report form; MRI = magnetic resonance imaging; N’pathol = neuropathology; CRO = contract research organization.