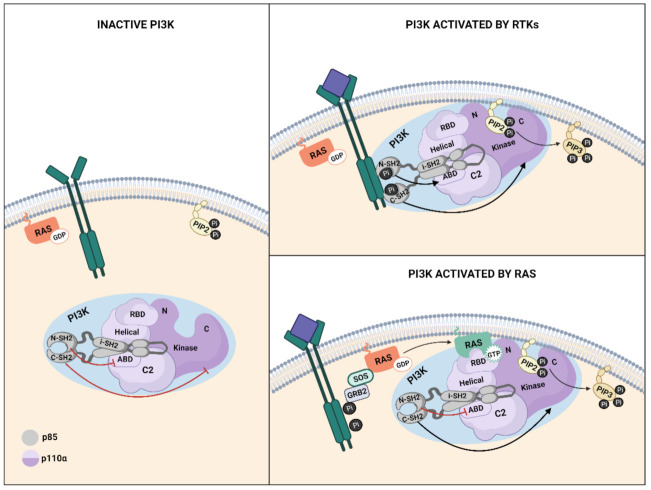
Figure 3.
PI3K activation. Under unstimulated conditions, interactions between the regulatory and the catalytic subunit keep PI3Ks in the inactive state. The nSH2 and iSH2 (coiled portion) domains of p85 are the minimal fragment of the regulatory subunit required for full inhibition of p110α lipid kinase activity. In PI3K activation by RTKs, binding of p85 to phosphorylated RTKs disrupts the inhibitory contact between the regulatory and the catalytic subunit, which generates conformational changes in p110α for substrate catalysis. In PI3K activation by Ras, the interaction of Ras with p110 displaces nSH2 and iSH2 in p85 from the p110α subunit, facilitating the full activation of PI3Kα.