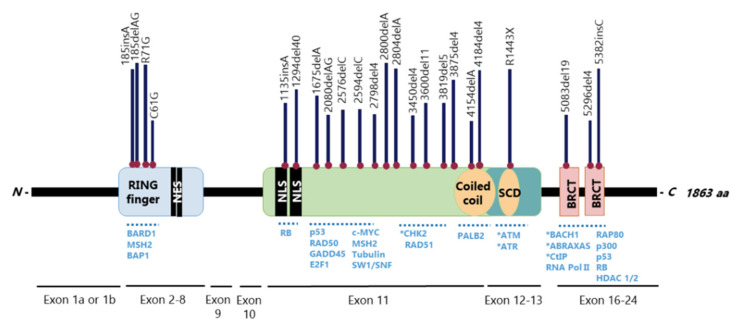
Figure 2.
Structural organization of BRCA1 with respective interacting proteins and most prevalent mutations. Full length BRCA1 contains two conserved domains at its termini: N-terminus containing a really interesting new gene (RING) domain (exons 2–8) and tandem BRCA1 C-terminus (BRCT) repeats (exons 16–24). The BRCA1 RING domain interacts with BRCA1-associated RING domain (BARD1), mutS homolog 2 (MSH2) and the ubiquitin hydrolase BRCA1-associated protein 1 (BAP1). The BRCT domains form a phospho-binding module, recognizing a phospho-SPxF motif that allow BRCA1 A complex subunit (ABRAXAS), BRCA1-associated C-terminal helicase (BACH1) and C-terminal-binding interacting protein (CtIP) to physically interact with BRCA1. A number of other proteins may also bind to BRCA1 C-terminus, as p53, p300, receptor-associated protein 80 (RAP80), retinoblastoma (Rb), RNA polymerase II and histone deacetylases (HDAC1/2). Several proteins bind to exon 11, as Rb, E2F transcription factor 1 (E2F1), growth arrest and DNA damage-inducible 45 (GADD45), p53, checkpoint kinase 2 (Chk2), RAD51, SWItch/Sucrose non-fermentable (SWI/SNF), among others. The interaction of BRCA1 with partner and localizer of BRCA2 (PALB2) and BRCA2 is mediated by the coiled-coil domain. The serine cluster domain (SCD) contains multiple ataxia-telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related protein (ATR) phosphorylation sites. BRCA1 contains two nuclear localization signals (NLS) and two nuclear export signals (NES). In the upper representation, the location and frequency of reported cases with BRCA1 pathogenic mutations are shown, including the most frequent C61G, 185delAG and 5382insC. (*) Phosphorylated proteins.