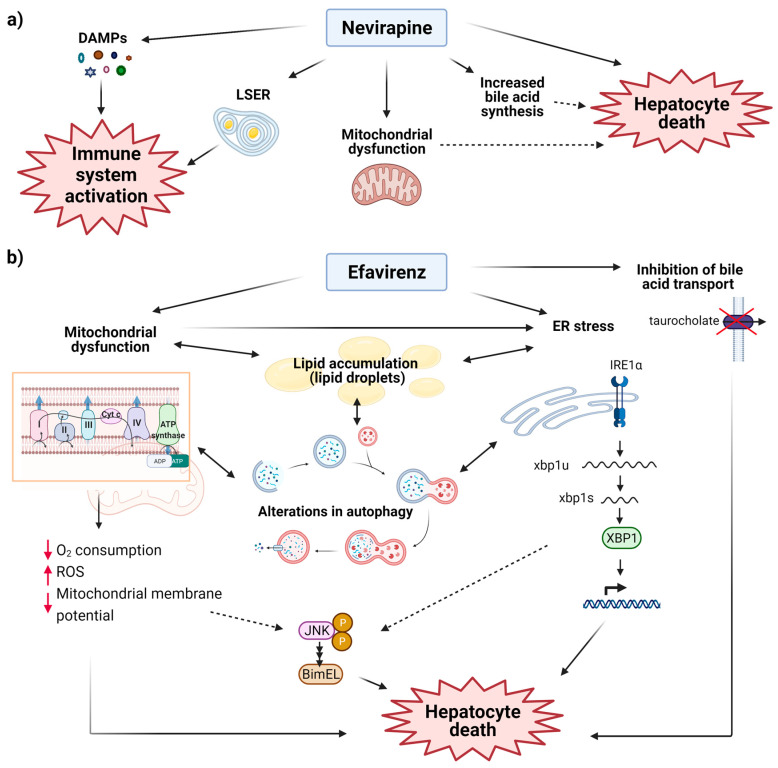
Figure 3.
Mechanisms of NNRTI-induced liver toxicity suggested by in vitro and in vivo studies for (a) Nevirapine (NVP) and (b) Efavirenz (EFV). NVP leads to the release of danger-associated molecular pattern molecules (DAMPs) by hepatocytes, and to the formation of hepatocytic inclusions composed of lipid droplets surrounded by smooth endoplasmatic reticulum cisternae (LSER), which are deposited in sinusoids. These two events presumably contribute to triggering the immune response. NVP is also proposed to induce mitochondrial dysfunction and to increase bile acid synthesis, while at supra-therapeutic concentrations, this drug induces hepatocyte apoptosis. EFV inhibits complex I of the electron transport chain, which diminishes oxygen consumption and mitochondrial membrane potential, and increases reactive oxygen species (ROS) production, resulting in mitochondrial dysfunction. Moreover, this drug produces ER stress, since it activates inositol-requiring 1α (IRE1α) and, consequently, IRE1α-catalyzed splicing of X-box binding protein 1(XBP1) mRNA. EFV-induced mitochondrial dysfunction and ER stress are thought to be interconnected, to alter autophagic flux and to promote lipid accumulation inside hepatocytes. c-Jun N-terminal kinase (JNK) pathway is also activated in response to EFV, possibly related to alterations in mitochondrial and ER function. In addition, EFV also inhibits bile acid transport. All these events have been shown to promote hepatocyte death.