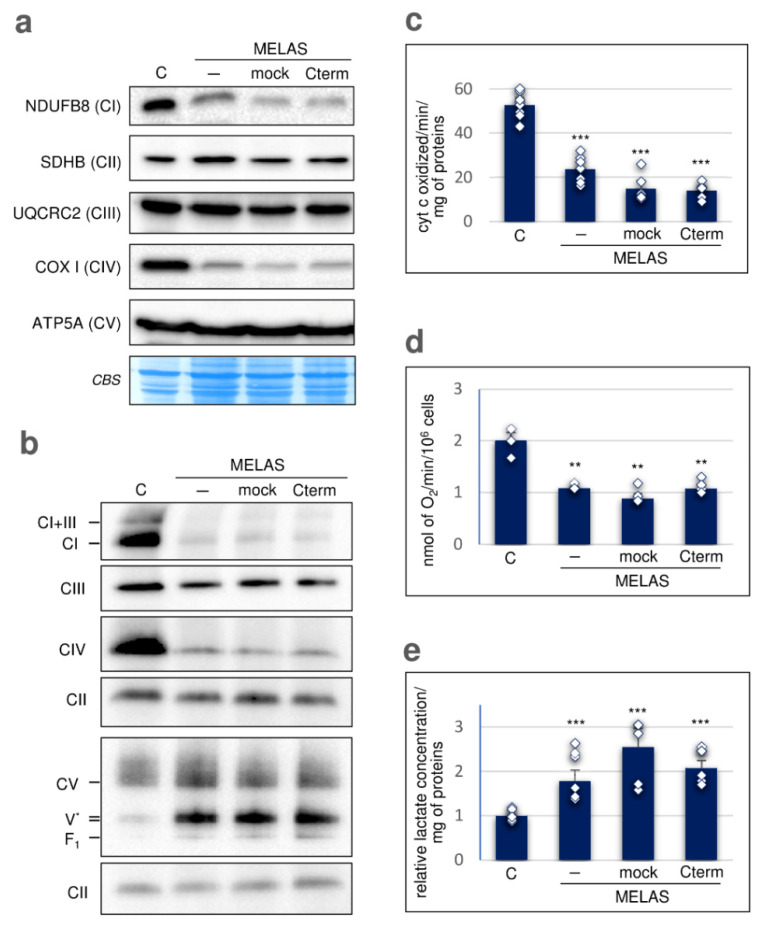
Figure 4.
Cterm does not improve mitochondrial bioenergetic competence. (a) SDS-PAGE western blot analysis of representative subunits of complex I (NDUFB8), II (SDHB), III (UQCRC2), IV (COX I, mtDNA-encoded) and V (ATP5A or alpha subunit). Immunoblot was carried out using total OXPHOS antibody cocktail (Abcam). Coomassie blue staining (CBS) of the membrane was used as transfer control. (b) Blue-native PAGE western blot analysis of the respiratory chain assembled complexes in cybrid cells. Holoenzyme complexes and sub-complexes were visualized by single primary antibodies, as follows: NDUFA9 for complex I, UQCRC2 for complex III, COX I for complex IV, ATP5A for complex V, and SDHA for complex II (used as loading reference). F1-ATPase domain alone (F1) and with several c-subunits (V*) are also indicated. (c) Measurement of cytochrome c oxidase (complex IV) activity in whole cybrid cells. Enzymatic activity is expressed as nmol of cytochrome c oxidized/min per mg of proteins. (d) Mitochondrial oxygen consumption rate in intact cybrids. Each value is expressed as nmol of O2/min per 106 cells. (e) Extracellular lactate levels were measured in cell growth media, normalized to total cellular proteins and expressed as fold change relative to control cells (fixed as 1-value). In panels (c–e), results are presented as the mean ± S.D. and statistical analyses were performed on at least three independent biological replicates using two-tailed Student’s t test; asterisks (**, p < 0.01; ***, p < 0.001) indicate the significance of variations in MELAS samples with respect to the control. Individual data points are depicted by white diamond-shaped dots. For all the panels, cells were as previously specified.