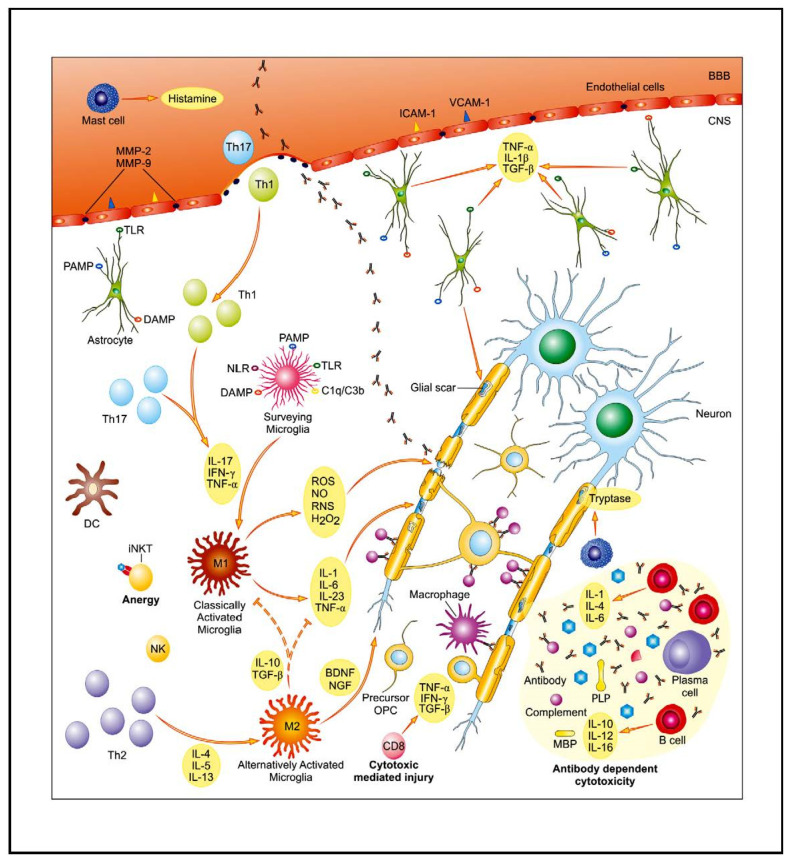
Figure 1.
The core of multiple sclerosis (MS) background is associated with disturbed, autoreactive activity of both the innate and adaptive immunological system. As a result of complex interplay between genetic and environmental factors, pools of auto-reactive T cells are activated and enter the central nervous system (CNS) through the disrupted blood–brain barrier (BBB). Their entry is facilitated, i.e., by enhanced expression of endothelial adhesion molecules (ICAM-1, VCAM-1) and matrix metalloproteinases (MMP-2, MMP-9). An activation of glial cells further contributes to pro-inflammatory properties of the CNS environment. Multiple mechanisms of immune-mediated injury of myelin and axons have been postulated: cytokine-mediated damage, digestion of surface myelin antigens by macrophages, antibody-dependent and complement-mediated cytotoxicity, and direct cytotoxic attack by CD8+ T cells. Parallel to inflammatory activity, there is slowly expanding neurodegenerative injury with axonopathy. The main contributing factors include: toxic metabolites (ROS, NO, RNS), mitochondrial and peroxysomal dysfunction with energetic deficit as well as disturbed ionic balance, and emerging pro-apoptotic activity. Abbreviations: BDNF—brain-derived neurotrophic factor, DAMP—danger associated molecular pattern, DC—dendritic cell, IFN-γ—interferon γ, IL—interleukin, iNKT cells—invariant natural killer T cells, MBP—myelin basic protein, NGF—nerve growth factor, NLR—NOD-like receptors, PAMP—pathogen-associated molecular pattern, TGF-β—transforming growth factor β, Th—T helper, TLR—Toll-like receptor, TNF-α—tumor necrosis factor α. Adapted from [5].