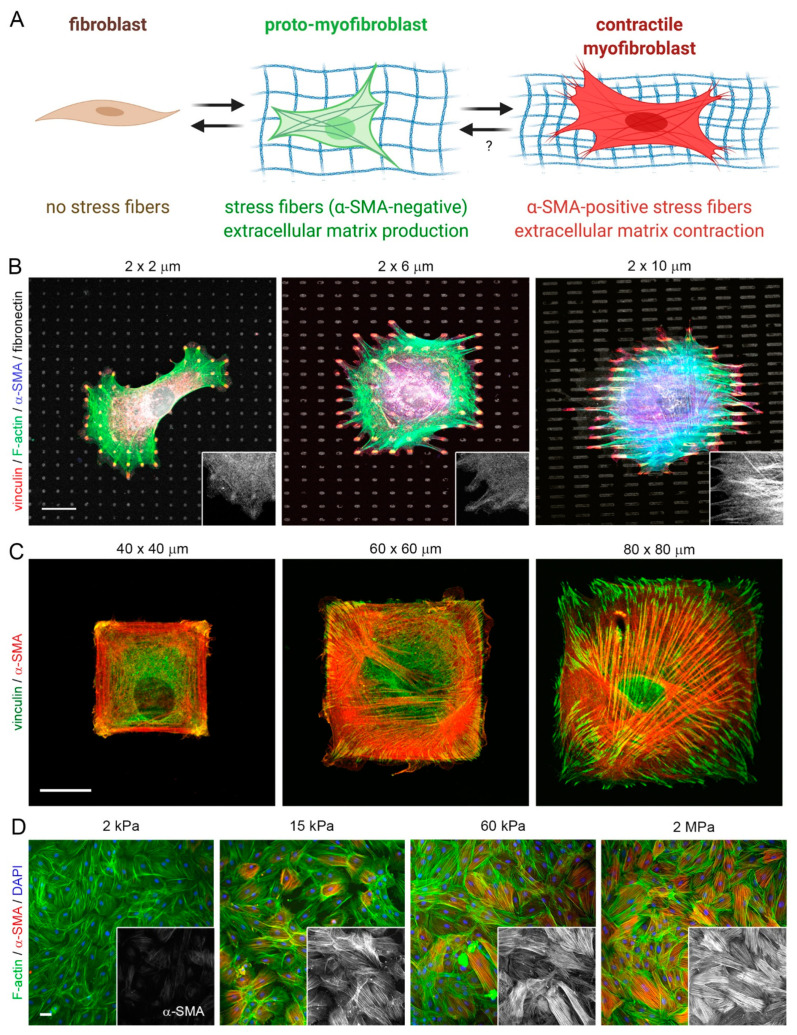
Figure 4.
Control of fibroblast-to-myofibroblast activation by adhesion patterns and substrate stiffness. (A) Activation of non-contractile (‘quiescent’) tissue fibroblasts into highly contractile, α-SMA stress fiber forming myofibroblasts passes over consecutive activation stages, one of which is characterized by α-SMA-negative stress fibers. This so-called pro-myofibroblast produces extracellular matrix and is the prevalent fibroblast phenotype in conventional cell culture. Myofibroblast activation stages can be controlled in culture by altering substrate adhesion patterns or stiffness. (B–D) Myofibroblasts from various origins were seeded onto different 2D culture substrates, followed by immunostaining for various proteins, color-coded as indicated. Acute incorporation of the myofibroblast marker α-SMA into stress fibers is increasing as a function of (B) the size of small fibronectin attachment islets that accommodate single focal adhesions, (C) area of large fibronectin adhesive islands that house single cells, and (D) stiffness of fibronectin-coated silicone elastomer substrates (elastic modulus in kPa). All these factors directly affect the ability of myofibroblasts to develop intracellular stress (actomyosin contractility). Scale bars: 20 µm. Scheme produced with Biorender.com.