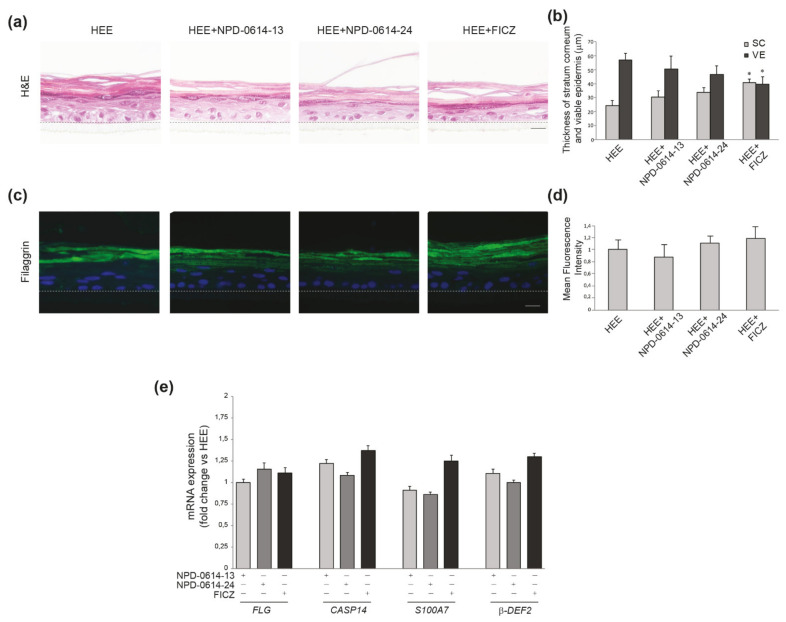
Figure 6.
NPD-0614-13 and NPD-0614-24 safety profile on human epidermal equivalents (HEEs). (a) Hematoxilin–eosin staining of HEEs cultured in presence or absence of NPD-0614-13, NPD-0614-24 (25 μM) and FICZ (100 nM) for 72 h. Bar: 50 μm. (b) Morphometric analysis of the stratum corneum (SC) and the viable epidermal layers (VE). The thickness of SC and VE was expressed as mean value ± SD (* p < 0.05 vs. untreated HEEs). (c) Representative immunofluorescence images after using filaggrin antibody. Nuclei were counterstained with DAPI. Bar: 20 μm. (d) Quantitative analysis of fluorescence intensity was expressed as mean value ± SD. Dashed black/white lines represent the junction between the basal layer and the membrane of the insert. (e) Quantitative real time PCR analysis of filaggrin, caspase-14, S100A7 and beta-defensin-2 in HEEs, in the presence of absence of NPD-0614-13, NPD-0614-24 (25 μM) and FICZ (100 nM) for 72 h. All mRNA values were normalized against the expression of GAPDH and were expressed relative to untreated HEEs.