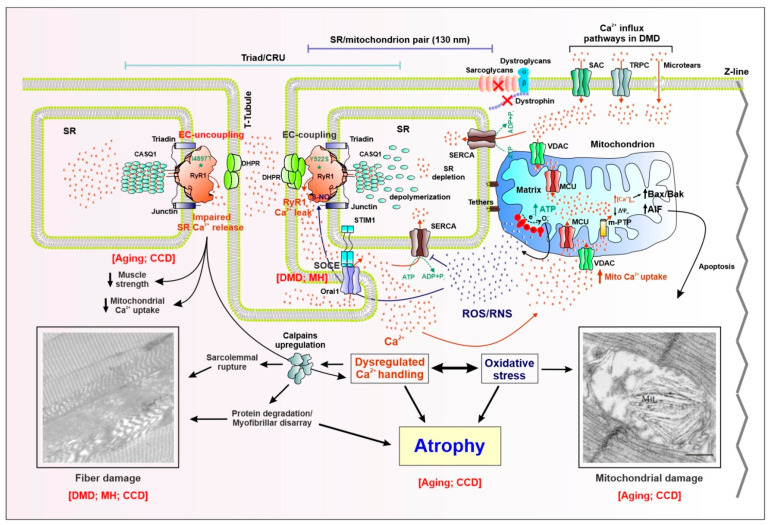
Figure 1.
Schematic model showing proposed molecular mechanisms for altered Ca2+ signaling, mitochondrial function, and muscle fiber damage/atrophy in skeletal muscle disease and aging. Skeletal muscle contraction relies on a rapid and massive release of Ca2+ from the sarcoplasmic reticulum (SR) terminal cisternae upon depolarization of the sarcolemma. Excitation–contraction (EC) coupling, the process whereby an action potential in the surface membrane is converted into Ca2+ release from SR, is mediated by a functional coupling between the dihydropyridine receptor (DHPR) voltage sensor in the transverse tubule (T-tubule) membrane and the type-1 ryanodine receptor (RyR1) Ca2+ release channel in the SR. The fundamental structure that mediates EC coupling is the Ca2+ release unit (CRU), or “triad”, which is composed of a central T-tubule flanked by adjacent junctions with two SR terminal cisternae. Besides DHPR and RyR1, other proteins participate in EC coupling: triadin and junction in the SR membrane, and calsequestrin-1 (CASQ1), the Ca2+-binding protein resident in the SR lumen. The ATP needed for muscle contraction is primarily generated within the mitochondria during aerobic respiration. In fast-twitch fibers, most mitochondria are located on the Z-line side of the triad, closely associated with the terminal SR cisternae via small (~8–10 nm) electron dense bridges termed “tethers”. As a result of this structural linkage, the average minimal distance between the RyR1 (site of Ca2+ release during EC coupling) and the outer membrane of the adjacent mitochondrion is ~130 nm. Right: Myoplasmic Ca2+ overload is the result of: (i) excessive SR Ca2+ release, due to gain-of-function point mutations (e.g., Y522S) in RyR1 linked to muscle disorders such as malignant hyperthermia (MH) and central core disease (CCD), that enhance channel opening probability; (ii) enhanced Ca2+ influx via STIM1/Orai1-dependent store-operated Ca2+ entry (SOCE), as the result of reduced SR Ca2+ content (SR depletion), stretch-activated Ca2+ channels (SAC), transient receptor potential canonical channels (TRPC), and microtears. These Ca2+ influx mechanisms are upregulated in Duchenne muscular dystrophy (DMD) and MH (SOCE). The resulting increase in Ca2+ influx into the myoplasm promotes mitochondrial Ca2+ uptake through the mitochondrial Ca2+ uniporter (MCU), ultimately leading to mitochondrial Ca2+ overload that increases electron transport chain activity and excessive production of reactive oxygen and nitrogen species (ROS/RNS), which underlie oxidative stress. In turn, increased ROS/RNS levels oxidize/nytrosylate both RyR1, which further enhances SR Ca2+ release channel opening, and the sarco/endoplasmic Ca2+ ATPase (SERCA), which reduces SR Ca2+ reuptake. The resulting accumulation of Ca2+ in the myoplasm, together with increased oxidative stress, triggers a series of intracellular signaling pathways (e.g., calpains activation, reduced protein synthesis, and increased protein degradation) that lead to: (i) myofibrillar disarray, (ii) sarcolemmal rupture, (iii) structural alterations (e.g., contractures, cores), and (iv) mitochondrial damage. These alterations, together with increased apoptosis, triggered by mitochondrial Ca2+ overload via the activation of the Bax/Bak/AIF pathway, drive loss of muscle mass and atrophy. Left: EC uncoupling, due to the reduction of DHPR expression during aging or as the result of RyR1 loss-of-function point mutations (e.g., I4897T) linked to myopathies such as CCD, reduces electricallyevoked SR Ca2+ release that contributes to reduced muscle specific force production, disrupted mitochondrial structure/function, mitochondrial damage, and fiber structural alterations (e.g., formation of cores and myofibrillar disarray). The two pictures showing fiber and mitochondrial damage are modified from Michelucci et al., 2017 Oxid Med Cell Longev. 2017; 2017: 6936897, and Michelucci et al., 2017 Oxid Med Cell Longev. 2017; 2017: 6792694, respectively.