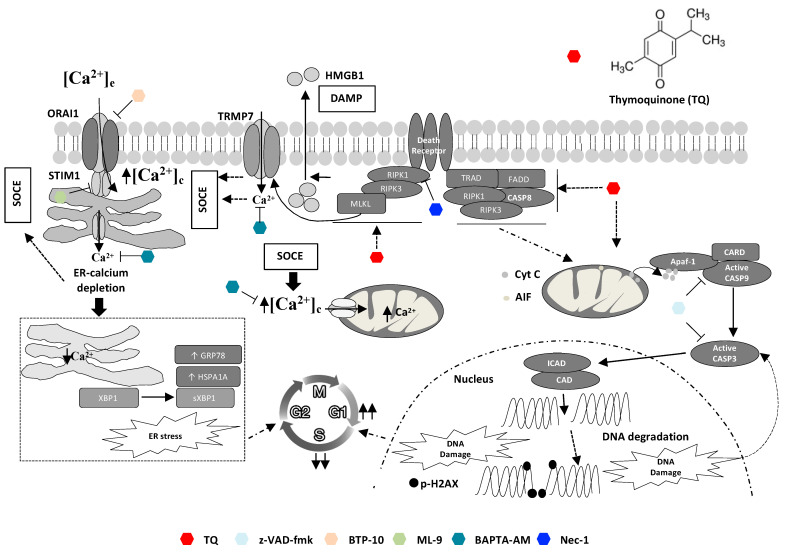
Figure 7.
Graphical summary of involved mechanisms in TQ-induced cell death. TQ promotes both apoptotic and non-apoptotic cell death modalities: an early and critical role of necroptosis and cytosolic calcium. Non-apoptotic cell death involving necroptosis signaling is the most critical cell killing mechanism underlying TQ-cytotoxicity. Necroptotic features appear early after TQ treatment as evidenced by an early increase in cytosolic calcium and latterly by extracellular release of HMGB1. Both BAPTA-AM, a cytosolic calcium chelator, and nec-1, a RIPK1 inhibitor, reversed TQ-induced non-apoptotic cell death. Cytosolic calcium increase during TQ treatment involves activation of SOCE after ER-calcium depletion, which also leads to ER stress as manifested by increase of GRP78, HSPA1A, and sXBP1; BTP-10 and ML-9, inhibitors of STIM1 and ORAI, respectively, blocked SOCE. Promotion of SOCE occurs more likely downstream to necroptosis initiation and is potentially mediated by TRMP7; nec-1 reduced TQ-induced SOCE. Apoptotic effect of TQ occurs through the intrinsic pathway as highlighted by cytochrome c release from the mitochondria and caspase-9/3 activations leading to PARP cleavage and potentially to DNA damage after DNA fragmentation by ICAD activated in turn by active caspase-3. The pan-caspase inhibitor z-VAD-fmk blocked caspase activity, but failed to reverse the cell death in most cells except the less sensitive cells. DNA damage and ER stress and their upstream inducers cooperate to TQ-induced cell cycle arrest.