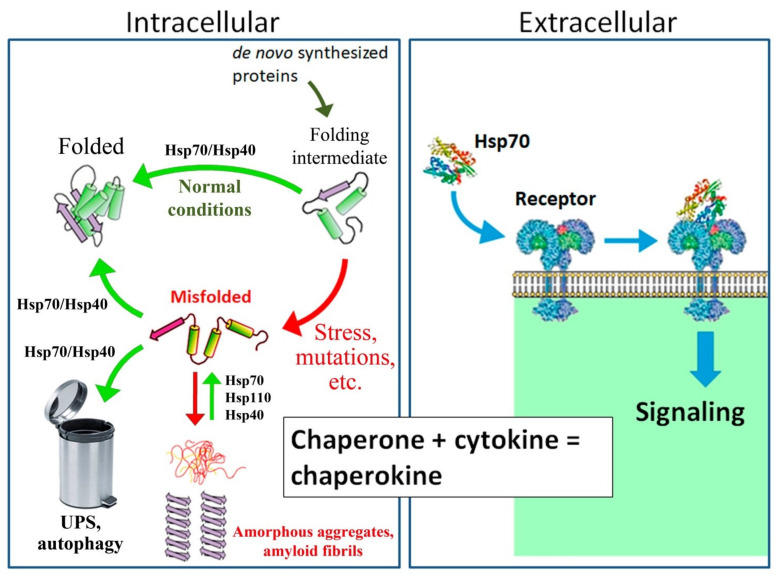
Figure 1.
Mechanism of action of intracellular and secreted proteins of the Hsp70 family. As an intracellular housekeeping protein, Hsp70, in cooperation with Hsp40 and other co-chaperones, folds and sorts newly synthesized proteins in cells under normal conditions. Under proteotoxic stress and in the case of certain mutations, misfolded proteins accumulate in the cytosol. Hsp70, in cooperation with co-chaperone Hsp40, restores the native conformation of partially denatured proteins and directs irreversibly damaged proteins to the ubiquitin–proteasome system (UPS) or lysosomes by autophagy. In addition, Hsp70 in combination with Hsp110 and Hsp40 promotes the dissolution of protein aggregates, such as α-synuclein [12,17,18,19,20]. As a secreted protein, Hsp70 is recognized by TLR2/4 and CD91 receptors and participates in the regulation of innate immunity, similar to classical cytokines; hence, Hsp70 is often called a “chaperokin” [21,22,23,24,25,26,27,28].