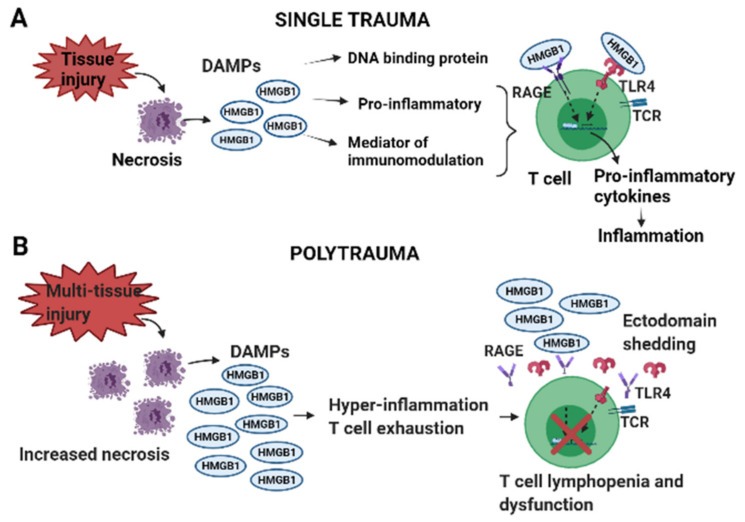
Figure 7.
Graphical illustration showing the effects of high mobility group box protein 1(HMGB1), a damage-associated molecular pattern (DAMP) in (A) single trauma and (B) polytrauma (PT). Several DAMPs such as HMGB1, mitochondrial DNA (mtDNA), S100 and other similar molecules are released from the cytosol of necrotic cells following injury and the damage of the cell membranes. Despite the importance of all DAMPs in mounting inflammation, here we shed light on the role of HMGB1 in PT due to its prominence as an early post-traumatic predictor marker [14,15]. Following injury circulating levels of HMGB1 are relatively lower in a single injury than in PT. HMGB1 mediates pro-inflammatory and immunomodulatory responses by binding to receptor for advanced glycation end products (RAGE) and toll-like receptor 4 (TLR4) on immune cells and triggers pro-inflammatory cascades in the surrounding cells the host must compensate for to maintain homeostasis. However, while inflammation is vital for regulating tissue homeostasis and repair if overburdened by the early surge of HMGB1, it can result in hyper-inflammation and immune cell exhaustion causing T cells to alter their phenotypes and undergo ectodomain shedding of RAGE and TLR4, leading to T cell lymphopenia and dysfunction.