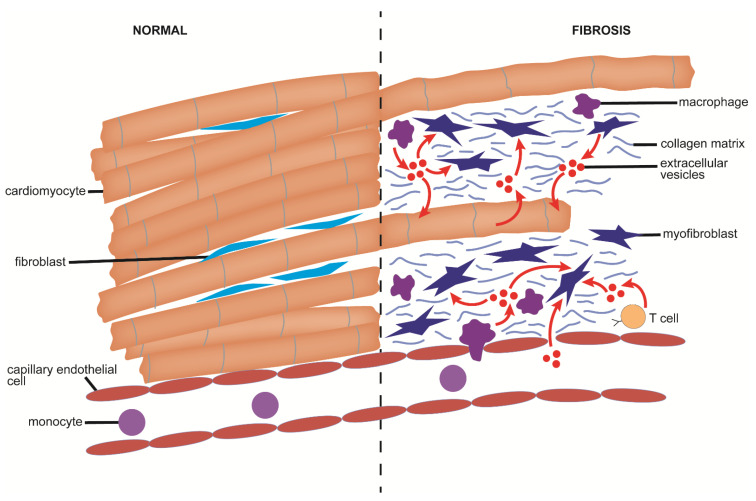
Figure 5.
Proposed EV pathways in the pathogenesis of cardiac fibrosis. Cardiomyocytes exposed to stress such as hypoxia produce EVs that promote mesenchymal transition, survival, and fibrogenesis in cardiac fibroblasts/myofibroblasts in part due to delivery of enriched EV cargo components that include Neat 1, miR-29b-3p, miR-30d-5p, miR-100-5p, miR181a, miR-21 and miR-208a, the latter two of which target, respectively, AKT-PTEN and Dyrk2. TNF-α-stimulated cardiomyocytes and cardiac fibroblasts produce EVs that are exchanged between them and which can promote increased oxidative stress by delivery of Nrf2-tartgeting miR-27a, -28-3p, or -34a, while profibrotic pathways in cardiac fibroblasts are promoted by delivery of fibroblast EVs enriched with Wnt3a or Wnt5a. High glucose-stimulated macrophages produce EVs that are enriched in HuR which is required for EV-stimulated expression of inflammatory or fibrogenic genes in cardiac fibroblasts. Macrophage EVs are also enriched in miR-155 which exerts, firstly, anti-proliferative and pro-inflammatory effects in cardiac fibroblasts by targeting Son of Sevenless 1/SOCS1 and, secondly, pyroptosis, hypertrophy and fibrosis in cardiomyocytes by its targeting of Foxo3a. Finally, age-related declines in HSP70 levels in serum EVs are associated with increased cardiac fibroblast proliferation. Only cells with a demonstrated role in EV production or response are shown; some of the depicted EV pathways are deduced from in vitro observations and have not been demonstrated in vivo. See text for details.