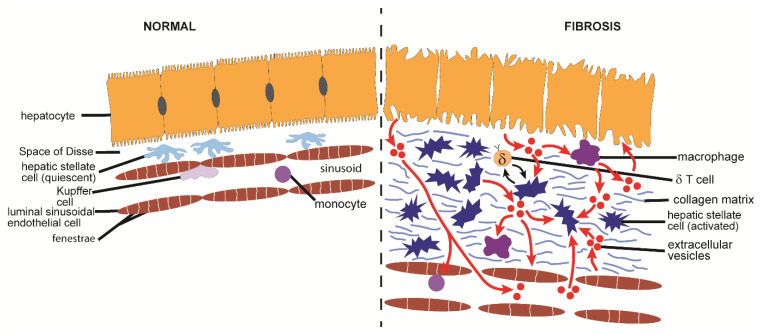
Figure 6.
Proposed EV pathways in the pathogenesis of hepatic fibrosis. Hepatocytes that are injured by exposure to hepatitis viruses, alcohol, or free fatty acids produce increased numbers of EVs due to activation of EV biogenic components (e.g., ROCK-1) and suppression of autophagy-associated late endosomes. These EVs drive activation and function in macrophages or HSC, or alternatively are released into the circulation. Production by macrophages of pro-inflammatory cytokines (e.g., IL-1β, IL-6) occurs upon interactions of hepatocyte EVs with TLR and DR5 as well as stimulation of NF-κB-activated NLRP3 inflammasomes. Hepatocyte EVs also stimulate TLR3 expression in HSC which causes HSC activation and drives an IL-17A positive feedback loop between HSC and δ T cells that exacerbates inflammation and fibrosis. HSC activation and fibrogenesis is directly stimulated by hepatocyte EVs and involves various mechanisms such regulation of PPARγ by EV miR-128-3p, -27b, -130b, Smad 7 by EV miR-27a, Nrld2 by miR-181, SOCS3 by EV miR-19a, TGF-β by miR-192, and miR-26b and its collagen 1α2 target by EV MALAT. HSC themselves release EVs that are enriched in PDGFα, bind to HSC integrins and heparan sulfate proteoglycans, and stimulate HSC migration, activation, and fibrogenesis. EVs produced downstream of activation of apoptosis signaling regulating kinase 1 or HIF-1 during HSC activation deliver GLUT1 and PKM2 to quiescent HSC, KC or LSECs and are pro-fibrogenic in part due to suppressed EV miR-30a levels and a proteomic cargo that is enriched for ECM-, proteasome- and collagen-associated components. AKT activation and migration in HSC is stimulated by EVs from liver sinusoidal endothelial cells while vascular endothelial cells undergo tube formation in response to VEGF-enriched EVs from fibrotic myofibroblasts and demonstrate enhanced adhesion to monocytes in the presence of integrin- β1-enriched EVs from lipotoxic hepatocytes. Activated macrophages produce miR-103-3p-eriched EVs that stimulate HSC activation and fibrogenesis via suppression of KLF4. EVs in serum or plasma from fibrotic patients activate HSC and are likely derived from injured hepatocytes (e.g., NAFLD, alpha-1 antitrypsin deficiency) or mast cells (e.g., systemic mastocytosis). Only cells with a demonstrated role in EV production or response are shown; some of the depicted EV pathways are deduced from in vitro observations and have not been demonstrated in vivo. See text for details.