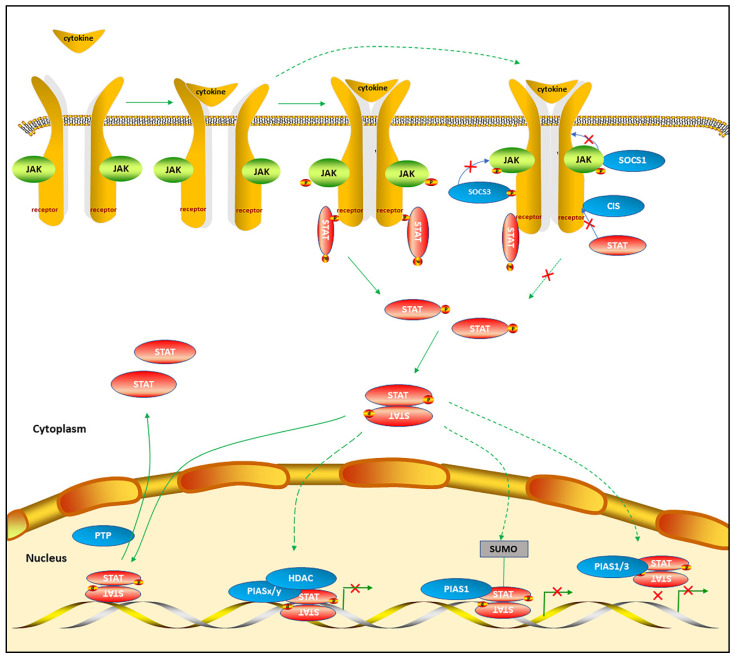
Figure 1.
Canonical STAT signaling pathways (solid lines) and STAT endogenous inhibitors (dashed lines). Cytokines binding to cognate receptors causes the cytokine receptors dimerization. The caspase of Janus kinases (JAKs) and STATs are phosphorylated and activated. Phosphorylated STATs dissociate from the receptors, form homodimers or heterodimers, and transfer to the nucleus. Intranuclear STATs bind to specific DNA elements and activate the transcription of cytokine-responsive genes. Protein tyrosine phosphatases (PTPs) dephosphorylate activated pSTATs in the nucleus and dissociate STATs. Then, STATs are exported into the cytoplasm. Suppressors of cytokine signaling (SOCS) are upregulated in response to ligands stimulation and inhibit JAK/STAT pathways through different mechanisms: SOCS1 binds to and inhibits JAKs; SOCS3 binds to JAK-proximal sites on cytokine receptors and inhibits JAK activities; CIS competes and blocks STATs binding to the docking site of cytokine receptors. Constitutive protein inhibitors of activated STATs (PIAS) interact with pSTAT dimers in the nucleus upon cytokine stimulation and inhibit STAT transcription activity through different mechanisms: PIAS1/3 binds to STATs and blocks DNA binding; PIASy/x recruit other co-repressor molecules such as histone deacetylases (HDACs) to inhibit STATs-mediated gene transcription; PIAS1 may promote the SUMOylation of STATs.