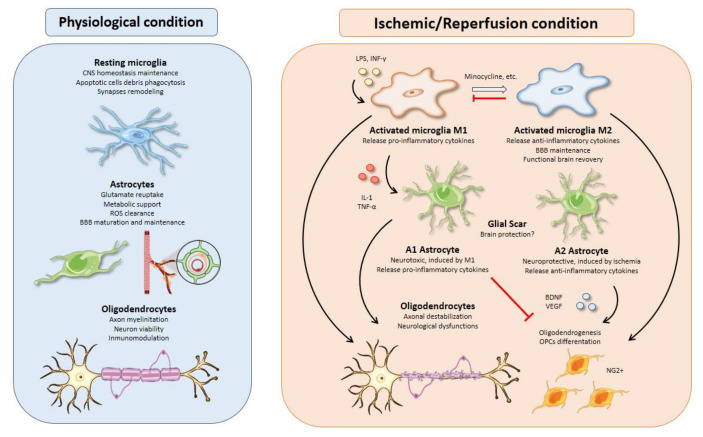
Figure 2.
Glial cell functions, responses, and interactions in physiological and I/R conditions. Microglial activation is one of the earliest events after brain ischemia. The inflammatory landscape generated in the ischemic brain by inflammatory cytokines, debris, or molecules released from dead cells triggers microglia activation. Activated microglia are typically divided into M1 and M2 phenotypes. M1 microglia present a pro-inflammatory profile releasing cytokines such as IL-1 or TNF-α that can polarize astrocytes toward a neurotoxic phenotype, aggravating the inflammatory response. On the other hand, M2-microglia release anti-inflammatory cytokines, sustain blood brain barrier (BBB) integrity, and stimulate oligodendrogenesis through oligodendrocyte progenitor cells’ (OPCs) differentiation into NG2+ cells, thus promoting functional recovery after brain ischemia. All therapeutic strategies based on microglia are focused on minimizing the effects of M1-microglia using drugs like minocycline to switch phenotype from M1 to M2. Besides, M2 microglia could promote an inhibition of M1 microglia. Activated astrocytes are usually classified into A1 and A2 phenotypes. In line with M1 microglia, A1 astrocytes present a neurotoxic profile releasing pro-inflammatory cytokines with inhibitory effects over oligodendrogenesis and OPCs’ differentiation. In contrast, A2 astrocytes have neuroprotective functions releasing anti-inflammatory cytokines and trophic factors, such as BDNF or VEGF with similar effects over oligodendrocytes as those of M2 microglia. Oligodendrocytes are especially sensitive to oxidative stress and excitotoxicity generated during brain ischemia. Demyelination affects neurons owing to axonal destabilization, generating neurological dysfunctions. Moreover, these events are aggravated in the presence of M1 microglia and A1 astrocytes. At the same time, trophic factors released by M2 microglia and A2 astrocytes increase oligodendrogenesis and OPCs’ differentiation in order to repair damaged white matter in the injury area.