Figure 2.
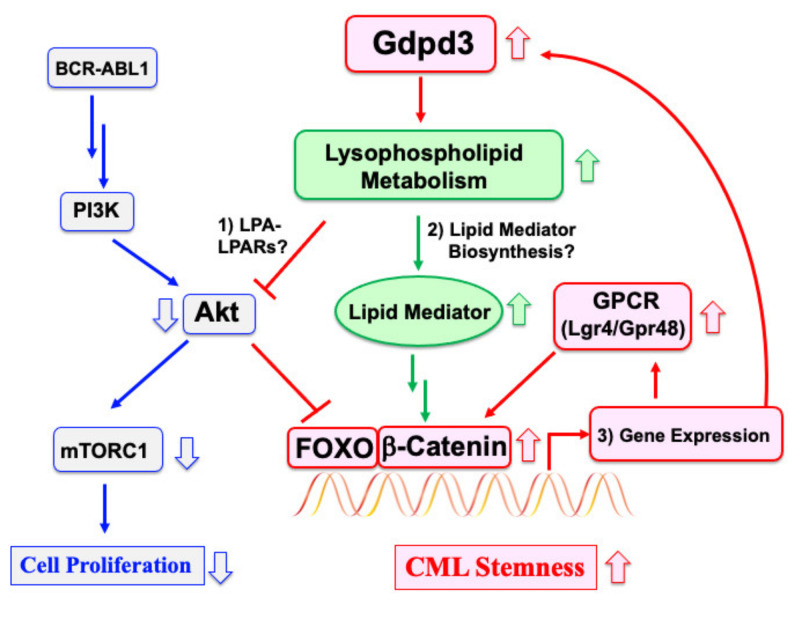
Regulation of CML stemness is independent of BCR–ABL1 oncogenic signaling. In the vast majority of mature CML cells, cell proliferation is driven by BCR–ABL1-mediated activation of the PI3K–Akt–mTORC1 signaling pathway. CML stem cells are able to maintain stem cell quiescence despite possessing the oncogene and so are TKI resistant. We have shown that Gdpd3 and lysophospholipid metabolism are essential for maintaining CML stem cell functions in vivo. Elevated lysophospholipid metabolism contributes to CML stemness by regulating an interaction between active Foxo3a and β-catenin (although the exact mechanism remains unclear). It is possible that Gdpd3-mediated lysophospholipid metabolism: (1) suppresses Akt via an LPA–LPARs pathway; (2) contributes to the biosynthesis of lipid mediators; and/or (3) participates in a gene expression program involving Gdpd3 and GPCRs by FOXO/β-catenin. Targeting any one of these elements of lysophospholipid metabolism specific to CML stem cells might provide fresh therapies to overcome disease relapse in many CML patients.